1. G. Johnson and colleagues have analyzed the MAP kinase cascade in which MEKK2 participates in mammalian...
Question:
a. HEK293 cells were transfected with a plasmid-encoding recombinant, tagged MEKK2, along with a plasmid-encoding MEK5 or a control vector that did not encode a protein (mock). Recombinant MEKS was precipitated from the cell extract by absorption to a specific antibody. The immunoprecipitated material was then resolved by polyacrylamide gel electrophoresis, transferred to a membrane,· and examined by Western blotting with an antibody that recognized tagged MEKK2. The results are shown in part (a) of the figure below. What information about this MAP kinase cascade do we learn from this experiment? Do the data in part (a) of the figure prove that MEKK2 activates MEKS or vice versa?
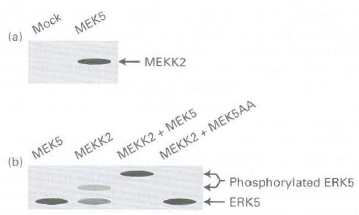
b. ERK5 is a MAP kinase previously shown to be activated when phosphorylated by MEK5. When ERK5 is phosphorylated by MEK5, its migration on a polyacrylamide gel is retarded. In another experiment, HEK293 cells were transfected with a plasmid encoding ERK5 along with plasmids encoding MEK5, MEKK2, MEKK2, and MEKS or MEKK2 and MEK5AA. MEK5AA is a mutant, inactive version of MEK5 that functions as a dominant-negative. Expression of MEK5AA in HEK293 cells prevents signaling through active, endogt:nuu~ .MEK5. Lysates of transfected cells were analyzed by Western blotting with an antibody against recombinant ERK5. From the data in part (b) of the figure, what can we conclude about the role of MEKK2 in the activation of ERK5? How do the data obtained when cells are cotransfected with ERK5, MEKK2, and MEKSAA help to elucidate the order of participants in this kinase cascade?
2. Scaffold proteins can segregate different MAPK signaling pathways that share common components. In the yeast mating pathway, the MEK (MAPKK) Ste7 phosphorylates and activates the Fus3 :VIAPK, whereas Ste7 phosphorylates and activates the Kssl MAPK in the starvation pathway. The mating pathway is activated by mating factor receptor activation of a G protein; Gp-y recruits the Ste5 scaffold protein and the Ste 1 l, Ste7, and Fus3 components of the kinase cascade. Mutation of the Ste5 binding sites for Stell and Ste7 disrupts rhe mating response, clearly demonstrating the importance of Ste5 for tethering the kinases together. Mutation of the Fus3 binding site on Ste5 gave a more complicated response, suggesting that Ste5-Fus3 interaction may involve more than just tethering. This possibility was investigated with yeast proteins expressed as recombinant proteins in bacteria or insect cells and then purified (see Good et al., 2009, Cel/136:1085-1097).
a. A fluorescence quenching assay was used to measure the activity of Fus3 and Kssl MAP kinases using a substrate peptide that can be phosphorylated by both kinases. Phosphorylated peptide binds to gallium coupled to fluorescence beads and quenches the fluorescence. The rate of fluorescence quenching (loss of fluorescence) corresponds to the Fus3 or Kssl kinase activity. Results of Ste7 phosphorylation and thereby activation of Kssl and Fus3 in the presence and absence of Ste5 arc shown below:
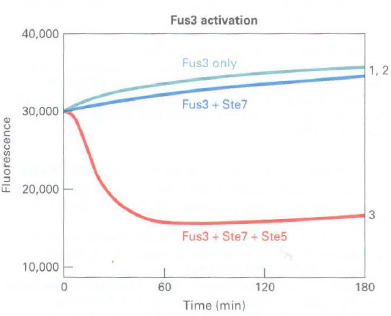
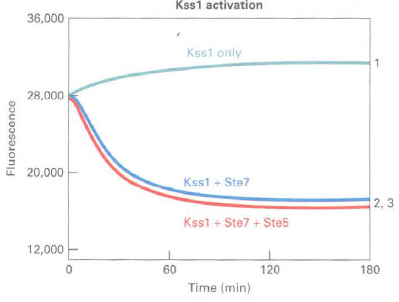
Quenching curves:
1. Fus3 or Kss l alone (control)
2. Fus3 or Ks!>l + Ste7
3. Fus3 or Kss l + Ste7 + Ste5
Is Ste5 required for Ste7 activity? Does Ste7 activate Fus3 and Kssl equivalently? What does the effect of the presence or absence of Ste5 in the assay tell you about Ste7 phosphorylation and activation of Fus3 and Kss 1?
b. The protein sequences of Fus3 and Kssl are 55 percent identical, but each has a unique so called MAPK insertion loop near the activation domain that is phosphorylated by Ste7. Mutation of an isoleucine residue in the Fus3 insertion loop or replacement of the Fus3 insertion loop with the equivalent region of Kss1 both yield curves similar to Kssl curve 2. What does this suggest about Fus3 and Kssl as substrates for Ste7 and the role of Ste5 in stimulating Ste7 phosphorylation of Fus3?
Step by Step Answer:

Molecular Cell Biology
ISBN: 978-1429234139
7th edition
Authors: Harvey Lodish, Arnold Berk, Chris A. Kaiser, Monty Krieger, Anthony Bretscher, Hidde Ploegh, Angelika Amon, Matthew P. Scott





