Figure 16.17 shows that atomic level resolution is only attainable in the repulsive portion of the tipsurface
Question:
Figure 16.17
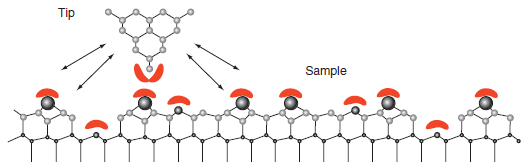
Transcribed Image Text:
Tip Sample
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The observation shows that the repulsive part ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Why is atomic level resolution obtained on pentacene in the AFM mode as shown in Figure 16.18, but not in the STM mode? Figure 16.18 1.3 A OA +1Hz -2Hz D 20A 5A -7Hz. -5Hz.
-
The DNA double helix (Figure 24.30) at the atomic level looks like a twisted ladder, where the "rungs" of the ladder consist of molecules that are hydrogen-bonded together. Sugar and phosphate groups...
-
The spent fuel elements from a fission reactor are much more intensely radioactive than the original fuel elements. (a) What does this tell you about the products of the fission process in...
-
What is your debt payments-to-income ratio if your debt payments total $684 and your net income is $2,000 per month?
-
Use the graph to find the projection of u onto v. (The terminal points of the vectors in standard position are given.) Use the formula for the projection of u onto v to verify your result. 1. 2. 3....
-
Import and chart monthly Bitcoin prices for the years 2014 to the present.
-
What have been some of the major efforts to specify human needs and values? You do not have to describe them or remember them in detail, but what are some of the most interesting and important points...
-
A firm pays a $1.50 dividend at the end of year one (D1), has a stock price of $155 (P0), and a constant growth rate (g) of 10 percent. a. Compute the required rate of return (Ke). Indicate whether...
-
The following is the balance sheet data of Johnson &Johnson company. Preferred Stock - SR300,000 Common Stock - SR250,000 Additional Paid in Capital: Additional Paid in Capital - Preferred Stock...
-
An experiment was conducted to test the efficacy of chloromycetin in checking typhoid. In a certain hospital chloromycetin was given to 285 out of the 392 patients suffering from typhoid. The number...
-
The reflection probability from a step potential was calculated for E > V 0 in Section 16.5. Is Equation (16.18) valid for E < V 0 ? What information can you extract from Figure 16.1 that will allow...
-
Why were quantum dots emitting in the near infrared region used for the surgery experiment shown in Figure 16.25? Figure 16.25 Color video 5 min post-injection NIR fluorescence 5 min post-injection...
-
What services might a modern full-service advertising agency offer a large business-to-business advertiser?
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
Solve each inequality. Graph the solution set, and write it using interval notation. VI -12 -6x + 3 15
-
6. (Potential Energy and Conservation of Energy) What should be the spring constant k of a spring designed to bring a 1200-kg car to rest from a speed of 95 km/h so that the occupants undergo a...
-
Molecular orbital calculations based on semi-empirical, ab initio, and DFT methods describe the spectroscopic properties of conjugated molecules better than simple Hckel theory. (a) Use the...
-
Use an appropriate semi-empirical method to compute the equilibrium bond lengths and standard enthalpies of formation of (a) ethanol, C 2 H 5 OH, (b) 1,4-dichlorobenzene, C 6 H 4 Cl 2 . Compare to...
-
Use appropriate electronic structure software and basis sets of your or your instructors choosing, perform self-consistent field calculations for the ground electronic states of H 2 and F 2 ....
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...
The European Anarchy Europes Hard Road Into High Politics 1st Edition - ISBN: 8716133366 - Free Book

Study smarter with the SolutionInn App


