Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25C.
Question:
Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25°C. Use data in Tables B.1 and B.11 to calculate the standard heat of formation of the solution in kJ/mol H2SO4 relative to the solute elements and water, and the total enthalpy of the solution relative to the same reference conditions.
Table B.11
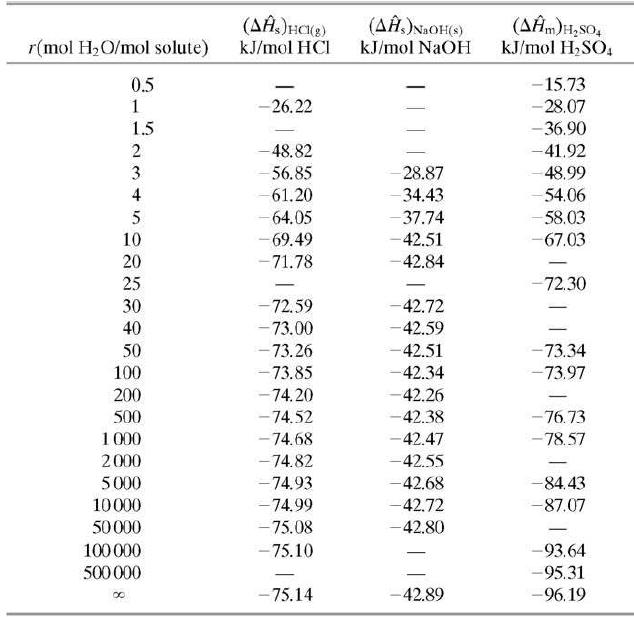
Transcribed Image Text:
(AĤ.)NaOH(s) kJ/mol NaOH (AĤm)H,sO, kJ/mol H,SO, r(mol H2O/mol solute) kJ/mol HCI 0.5 -15.73 -26.22 -28.07 1.5 - 36.90 -48.82 -41.92 28.87 - 34.43 - 37.74 3 56.85 48.99 4 61.20 54.06 - 64.05 - 69.49 - 58.03 10 -42.51 -67.03 20 -71.78 42.84 - 25 -72.30 30 72.59 -42.72 - 73.00 - 73.26 - 73.85 -42.59 -42.51 -42.34 40 50 -73.34 100 -73.97 200 -74.20 -42.26 - 74.52 -74.68 - 76.73 - 78.57 500 -42.38 1 000 2000 -42.47 - 74.82 -42.55 5000 -74.93 -42.68 -84.43 10000 -74.99 -42.72 -87.07 50000 - 75.08 -42.80 100 000 - 75.10 -93.64 -95.31 -96.19 500 000 - -75.14 -42.89
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)

Answered By

Nicole omwa
Being a highly skilled tutor with at least 5 years of tutoring experience in different areas, I learned how to help diverse learners in writing drafts of research papers, actual research papers and locate credible sources. My assurance is built upon my varied knowledge of a variety of subjects. Furthermore, my involvement and interaction with numerous learners of all levels has allowed me to understand my clients' specific demands. Ultimately, this has aided me in being a better coach to learners to better their grades. Essentially, my responsibilities as a tutor would include:
Teaching abilities that assist pupils in enhancing their academic performance
Personal interaction with learners to make them understand abstract concepts
Inducing new skills and knowledge into their academic journeys
Fostering individual reflection, and independent and critical thinking
Editing and proofreading
Because I am constantly available to respond to your queries, you may decide to rely on me whenever you require my assistance. As an assurance, my knowledge skills and expertise enable me to quickly assist learners with different academic challenges in areas with difficulty in understanding. Ultimately, I believe that I am a reliable tutor concerned about my learner's needs and interests to solve their urgent projects. My purpose is always to assist them in comprehending abstract schoolwork and mastering their subjects. I also understand that plagiarism is a severe offense and has serious ramifications. Owing to this, I always make it a point to educate learners on the numerous strategies to have uniquely unique solutions. I am familiar with the following formatting styles:
MLA
APA
Harvard
Chicago
IEEE
Communication is always the key in every interaction with my learners. Hence, I provide timely communication about the progress of assigned projects. As a result, I make sure that I maintain excellent communication with all of my clients. I can engage with all of my customers more effectively, assisting them with their unique academic demands. Furthermore, I attempt to establish a solid working relationship with my leaners I have exceptional abilities in the below areas;
Sociology
History
Nursing
Psychology
Literature
Health and Medicine
Chemistry
Biology
Management
Marketing
Business
Earth Science
Environmental Studies
Education
Being a teacher who aces in diverse fields, I provide various academic tasks, which include;
Academic Reports
Movie Reviews
Literature Reviews
Annotated bibliographies
Lab reports
Discussion posts
Dissertations
Case study analyses
Research proposals
Argumentative Essays
I guarantee you high-quality Papers!!!!!
5.00+
17+ Reviews
32+ Question Solved
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard heat of formation of the compound ICl (g) at 25oC. Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122 122221 25735 375859609...
-
A 10.0 mole% aqueous sulfuric acid solution (SG = 1.27) is to be titrated to neutrality at 25C with a 3.00-molar caustic soda (sodium hydroxide) solution (SG = 1.13): H 2 SO 4 (aq) + 2 NaOH (aq) Na 2...
-
A 5.00-wt% aqueous sulfuric acid solution (p = 1.03g/mL) flows through a 45-m long pipe with a 6.0cm diameter at a rate of 87L/min. (a) What is the morality of sulfuric acid in the solution? (b) How...
-
Let z(k) denote the k-year continuously compounded zero-coupon yield for the current term structure. You are given that z(1) = 0.035, z(2) = 0.041, z(3) = 0.045, z(4) = 0.049, z(5) = 0.051, z(6) =...
-
Is cognitive dissonance a good thing or a bad thing from an advertiser's point of view? Explain how and why advertisers should try to take advantage of the cognitive dissonance their consumers may be...
-
Determine whether each statement is true or false, and explain why. Knowing x, y, x 2 , xy, and n is sufficient for calculating the least squares line.
-
An object is 45 cm to the left of a lens, and the image is 25 cm to the left of the lens. What is the magnification?
-
A computer manufacturer is introducing a new product specifically targeted at the home market and wishes to compare the effectiveness of three sales strategies: computer stores, home electronics...
-
Account Titles and Explanation Debit Credit Accounts Receivable 492.700 Sales Revenue 492.700 (To record credit sale) Cost of Goods Sold 312.100 Inventory 312.100 (To record cost of merchandise sold)...
-
Atmospheric air at 1 atm, 32oC, and 95 percent relative humidity is cooled to 24oC and 60 percent relative humidity. A simple ideal vapor-compression refrigeration system using refrigerant-134a as...
-
Hydrogen is produced in the steam reforming of propane: The watergas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen: The reaction is carried out over...
-
Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25C. Use data in Tables B.1 and B.11 to calculate the standard heat of formation of the solution in kJ/mol H...
-
Determine Amys taxable income for 2019 if she has $40,000 of salary income, is single, and claims the standard deduction.
-
Question 9 (1 point) If the common law requires employees of a bar establishment to monitor a potentially intoxicated patron and to possibly make an effort to intervene if there is an indication the...
-
B. A velocity potential is given by the equation: Q = x-y 3. (10 pts) Short answer, What special characteristics of the velocity potential make it very useful in identifying a type of flow and...
-
Ted sold his Microsoft stock for $40,000 paying a commission of $800. He purchased the stock in 2004 for $8,000 and paid commission of $200. What is the recognized gain on the sale?
-
Liquid water at 80C and at 1atm flows through a heated pipe at a flow rate of 3.1 kg/s. It then leaves the pipe as steam. The water receives 9753840 J of heating from the pipe. Calculate the...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
Explain the connection between short-circuit Boolean expressions and normal-order evaluation. Why is cond a special form in Scheme, rather than a function?
-
Using thermodynamic data from Appendix 4, calculate G at 258C for the process: 2SO 2 (g) + O 2 (g) 88n 2SO 3 (g) where all gases are at 1.00 atm pressure. Also calculate DG8 at 258C for this same...
-
Provide at least five different acceptable IUPAC names for the following compound.
-
Compound A has molecular formula C 8 H 8 . When treated with excess Br 2 , compound A is converted into compound B, with molecular formula C 8 H 8 Br 2 . Identify the structures of compounds A and B....
-
In some circumstances, dehydrogenation is observed. Dehydrogenation involves the loss of two hydrogen atoms (the reverse of hydrogenation). Analyze each of the following dehydrogenation reactions and...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...

Study smarter with the SolutionInn App


