Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25C.
Question:
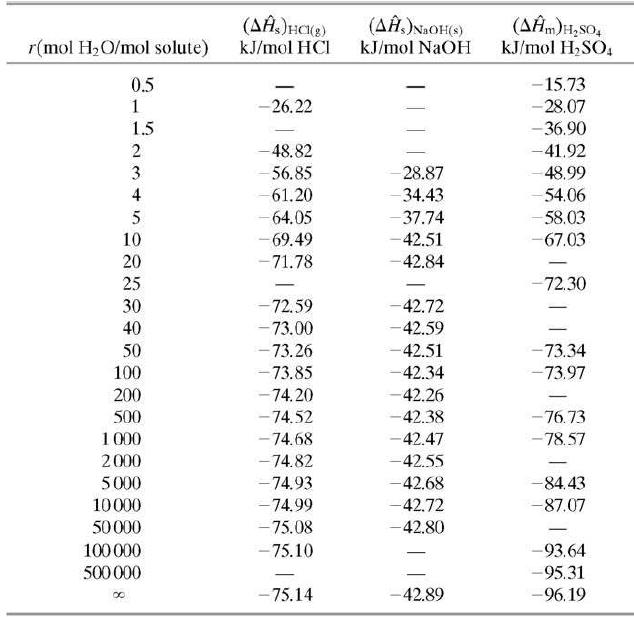
Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25°C. Use data in Tables B.1 and B.11 to calculate the standard heat of formation of the solution in kJ/mol H2SO4 relative to the solute elements and water, and the total enthalpy of the solution relative to the same reference conditions.
Table B.11
Transcribed Image Text:
(AĤ.)NaOH(s) (AĤm)H,sO, kJ/mol H,SO, r(mol H2O/mol solute) kJ/mol HCI kJ/mol NaOH -15.73 - 28.07 - 36.90 0.5 -26.22 1.5 2 -48.82 -41.92 3 56.85 28.87 -48.99 4 61.20 -34.43 -54.06 - 37.74 - 64.05 - 69.49 -71.78 58.03 10 -42.51 -67.03 42.84 20 25 -72.30 30 72.59 -42.72 -42.59 -42.51 40 - 73.00 - 73.26 - 73.85 - 74.20 50 -73.34 100 -42.34 -73.97 200 500 -42.26 -74.52 -42.38 -76.73 1 000 - 74.68 -42.47 - 78.57 2000 -74.82 -42.55 5000 -74.93 -42.68 -84.43 10000 -74.99 -42.72 -87.07 - 75.08 - 75.10 50000 -42.80 100000 -93.64 500 000 -95.31 - 75.14 -42.89 -96.19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
The standard heat of formation of the 1 00 m olar a que ous sulfur ...View the full answer

Answered By

SHINKI JALHOTRA
I have worked with other sites like Course Hero as a tutor and I have great knowledge on IT skills.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard heat of formation of the compound ICl (g) at 25oC. Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122 122221 25735 375859609...
-
A 10.0 mole% aqueous sulfuric acid solution (SG = 1.27) is to be titrated to neutrality at 25C with a 3.00-molar caustic soda (sodium hydroxide) solution (SG = 1.13): H 2 SO 4 (aq) + 2 NaOH (aq) Na 2...
-
A 5.00-wt% aqueous sulfuric acid solution (p = 1.03g/mL) flows through a 45-m long pipe with a 6.0cm diameter at a rate of 87L/min. (a) What is the morality of sulfuric acid in the solution? (b) How...
-
Raheem & Co. purchased a fixed asset on 1.4.2018 for Rs.2,50,000. Depreciation is to be provided @10% annually according to the Straight-line method. The books are closed on 31st March every year....
-
"In today's modern, highly educated society, there is simply no reason to separate men and women into different target segments. Gender just should not be an issue in the development of marketing and...
-
Determine whether each statement is true or false, and explain why. To find the x-intercept of the graph of a linear function, we solve y = (x) = 0, and to find the y-intercept, we evaluate (0).
-
An object is placed in front of a lens. The image is upright, 35 cm to the left of the lens, and is half as tall as the object. What is the focal length of the lens?
-
Pearson Brothers recently reported an EBITDA of $7.5 million and net income of $1.8 million. It had $2.0 million of interest expense, and its corporate tax rate was 40%. Calculate its charge for...
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. He has computed the cost and revenue estimates for each...
-
Draw the free-body diagram for the following problems. a) The lamp in Prob. 519. b) The rod in Prob. 520. c) The assembly in Prob. 521. d) The beam in Prob. 522. 0.3 m 0.4 m 0.15 m C B -0.5 m- G
-
Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25C. Use data in Tables B.1 and B.11 to calculate the standard heat of formation of the solution in kJ/mol...
-
Calcium chloride is a salt used in a number of food and medicinal applications and in brine for refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form...
-
Using the data presented in P10-17: a. Prepare a worksheet to develop a consolidated statement of cash flows for 20X3 using the direct method of computing cash flows from operations. b. Prepare a...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Write a program in your favorite imperative language that has the same input and output as the Scheme program of Figure 11.1. Can you make any general observations about the usefulness of Scheme for...
-
Match the following. Answers may be used more than once: Measurement Method A. Amortized cost B. Equity method C. Acquisition method and consolidation D. Fair value method Reporting Method 1. Less...
-
Predict whether each of the following compounds should be aromatic. a. b. c.
-
The cyclopropenyl cation has a three-membered ring that contains a continuous system of overlapping p orbitals. This system contains a total of two Ï electrons. Using a Frost circle, draw an...
-
Arrange each set of isomeric alkenes in order of stability. a. b.
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...
-
Use the following information: \ table [ [ Country , \ table [ [ Consumer Prices ] ] , Interest Rates,Current Units ( per US$ ) ] , [ Forecast , 3 - month, 1 - yx Covt Bond,, ] , [ 2 0 2 4 e ,...

Study smarter with the SolutionInn App


