Arrange each set of isomeric alkenes in order of stability. a. b.
Question:
a.
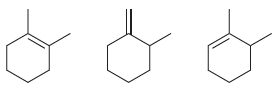
b.
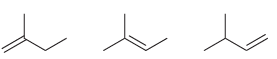
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (10 reviews)
a b di substit...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Acid-catalyzed dehydration of 2,2-dimethyl-1-hexanol gave a number of isomeric alkenes including 2-methyl-2-heptene as shown in the following formula. (a) Write a stepwise mechanism for the formation...
-
Using only the periodic table, arrange each set of atoms in order from largest to smallest: (a) K, Li, Cs; (b) Pb, Sn, Si; (c) F, O, N
-
Using only the periodic table, arrange each set of atoms in order of increasing radius: (a) Ba, Ca,Na; (b) Sn, Sb, As; (c) Al, Be, Si.
-
PLEASE, PLEASE use an Excel formula:1. Begin the formula with an = sign.2. Reference cells, instead of entering values.Example: =PV(H8+H9) A company borrowed money from a local bank. The note the...
-
Comment on the validity of the results of Atterberg limits on soils G and H.>
-
Tony has decided to end the Precision Computer Centres first year as of July 31, 2022. Below is an updated chart of accounts. Assignment 1. Journalize the closing entries. 2. Post the closing entries...
-
3. Under the modified accrual basis of accounting for a governmental unit, revenues should be recognized in the accounting period in which they a Are earned and become measurable b Are collected c...
-
Consider the McDonalds tracking survey presented in Branding Brief 8-1. What might you do differently? What questions would you change or drop? What questions might you add? How might this tracking...
-
I got C but I want to make sure its right. -Thanks The managerial accountant at Seaside Manufacturing reported the following data: 63.500 Units. Beginning WIP 75% for materials 45% for conversion...
-
Consider the half-wave rectifier circuit of Fig. 4.23(a) with the diode reversed. Let vS be a sinusoid with 10-V peak amplitude, and let R = 1 k. Use the constant-voltage-drop diode model with VD=...
-
Why is chlorine added to drinking water? What is the potential problem with adding chlorine to drinking water?
-
How do maximum containment levels and national emission limitations differ?
-
Can we infer that women are more likely than men to lose their jobs in the next 12 months (JOBLOSE: In the next 12 months how likely is it that you will lose your job or be laid off: 1 = Very likely,...
-
Problem 8-19 (Algo) Cash Budget; Income Statement; Balance Sheet [LO8-2, LO8-4, LO8-8, LO8-9, LO8- 10] Minden Company is a wholesale distributor of premium European chocolates. The company's balance...
-
Consider the unsteady flow of a fluid in the x direction through a control volume. The linear momentum of the fluid within the control volume is a function of time given by 200ti slug*ft/s, where t...
-
For a continuous uniform distribution with u = 0 and o = 1, the minimum is - V3 and the maximum is V3. For this continuous uniform distribution, find the probability of randomly selecting a value...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
Write using only positive exponents and then evaluate. Assume that all variables represent nonzero real numbers. -2 (1) 4x
-
Identify the most stable compound:
-
Ribose, an essential part of ribonucleic acid (RNA), has the following structure: (a) How many chirality centers does ribose have? Identify them. (b) How many stereo isomers of ribose are there? (c)...
-
On catalytic hydrogenation over a platinum catalyst, ribose (Problem 9.57) is converted into ribitol. Is ribitol optically active or inactive?Explain. CH2 Ribitol
-
Hydroxylation of cis-2-hutene with OsO4 yields 2, 3-butanediol. What stereochemistry do you expect for the product?
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


