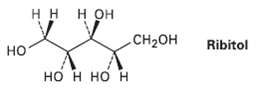
On catalytic hydrogenation over a platinum catalyst, ribose (Problem 9.57) is converted into ribitol. Is ribitol optically
Question:
On catalytic hydrogenation over a platinum catalyst, ribose (Problem 9.57) is converted into ribitol. Is ribitol optically active or inactive?Explain.
Transcribed Image Text:
нн нон CH2он Ribitol но но н но н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Ribitol is an optically inactive meso compound Catalyti...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
On catalytic hydrogenation over a rhodium catalyst, the compound shown gave a mixture containing cis-1-tert-butyl-4-methylcyclohexane (88%) and trans-1-tert-butyl-4 methylcyclohexane (12%). (a) What...
-
On catalytic hydrogenation over a rhodium catalyst, the compound shown gave a mixture containing cis-1-tert-butyl-4-methylcyclohexane (88%) and trans-1-tert-butyl-4-methylcyclohexane (12%). (a) What...
-
A triglyceride can be optically active if it contains two or more different fatty acids. (a) Draw the structure of an optically active triglyceride containing one equivalent of myristic acid and two...
-
Rand Medical manufactures lithotripters. Lithotripsy uses shock waves instead of surgery to eliminate kidney stones. Physicians' Leasing purchased a lithotripter from Rand for $2,000,000 and leased...
-
Is there a torque about the Moon's center of mass when the Moon's long axis is aligned with Earth's gravitational field? Explain how this compares with a magnetic compass.
-
A gas storage tank has a small leak. The pressure in the tank drops more quickly if the gas is hydrogen or helium than if it is oxygen. Why?
-
First, using the WVS dataset, run a regular regression model using IMMJOBS as the dependent variable and WORRYJOB as the independent variable. Then, imagine you didnt have the IMMJOBS variable, and...
-
Derive cash disbursements for dividends Johnson & Johnson, a pharmaceutical and medical products company, reported a balance in retained earnings of $26,571 million at the beginning of the year and...
-
The real risk-free rate is 1.75%. Inflation is expected to be 2.75% this year, 5.15% next year, and 2.7% thereafter. The maturity risk premium is estimated to be 0.05 (t - 1)%, where t = number of...
-
North Star prepared the following unadjusted trial balance at the end of its second year of operations ending December 31, 2012. Other data not yet recorded at December 31, 2012: a. Rent expired...
-
Ribose, an essential part of ribonucleic acid (RNA), has the following structure: (a) How many chirality centers does ribose have? Identify them. (b) How many stereo isomers of ribose are there? (c)...
-
Hydroxylation of cis-2-hutene with OsO4 yields 2, 3-butanediol. What stereochemistry do you expect for the product?
-
Below is the receipts and payments account of the Reygersdal Soccer Club for the period 1 July 20x6 to 30 June 20x7, and their statement of financial position as at 30 June 20x6: Additional...
-
In addition to the strongest military in the world, the United States wields enormous soft power. Define soft power. What factors make the United States powerful when it comes to soft power?
-
Tampa by the Bay Cardiology practice is experiencing long wait times for new patient appointments. Next available appointment is 30 days. The administrator has asked the practice manager to construct...
-
In 2013, Idalia Hernndez Ramos, a middle school teacher in Mexico, was a victim of cyber harassment. After discovering that one of her students tweeted that the teacher was a "bitch" and a "whore,"...
-
Your life couldn't be any better. You just accepted a new role as a senior consultant for a project management services firm in San Francisco, and the move is finally happening. You've got a great...
-
What are the two "engines" that drive earth's processes, how do they work (basically) and what are their energy sources? How do the "engines" influence and interact with the Earth Systems? (provide a...
-
What type of clientele are city clubs oriented toward? LO.1
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
A solution contains one or more of the following ions: Hg 2 2 + , Ba 2 + , and Fe 2 + . When you add potassium chloride to the solution, a precipitate forms. The precipitate is filtered off, and you...
-
Planteose, a carbohydrate isolated from tobacco seeds, can be hydrolyzed in dilute acid to yield one equiva-lent each of D-fructose, D-glucose, and D-galactose. Almond emulsion (an enzyme preparation...
-
A process called sizing chemically modifies the cellulose in paper. As a result, the paper resists wetting (and thus prevent inks from running). In addition, sizing leaves the paper in a slightly...
-
Explain with a mechanism why treatment of the 2- deoxy-2-amino derivative of o-glucose (n-glucosamine) with aqueous NaOH liberates ammonia. HOCH HO OH NH2 D-glucosamine
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


