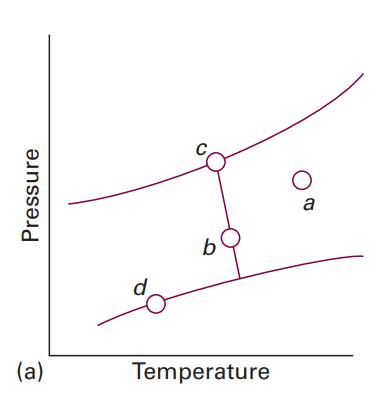
How many phases are present at each of the points marked in Fig. 4.1a? Data in Fig.
Question:
How many phases are present at each of the points marked in Fig. 4.1a?
Data in Fig. 4.1a?
Transcribed Image Text:
Pressure (a) d b Temperature O a
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
There are 3 phases present at any point marked in Fig 41a i...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Mr. Lee, clinic director claims that through new procedures he has reduced patient waiting time from present 55 minutes. Before implementing his procedures nationwide, the clinic owner, Ms. Groetken,...
-
Each of the points listed next represents an internal control that may be implemented within a companys accounting information system to reduce various risks. For each point, identify the appropriate...
-
Consider the following phase diagram. What phases are present at points A through H? Identify the triple point, normal boiling point, normal freezing point, and critical point. Which phase is denser,...
-
Carey Company is borrowing $200,000 for one year at 12 percent from Second Intrastate Bank. The bank requires a 20 percent compensating balance. What is the effective rate of interest? What would the...
-
How do firms record prior- period adjustments? .
-
Refer to the data in Exercise 16-2 for Weller Corporation. Data From Exercise 16-2: Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31...
-
Impact of paper color on exam scores. A study published in Teaching Psychology (May 1998) examined how external clues influence student performance. Introductory psychology students were randomly...
-
On January 1, 2015, Paro Company purchases 80% of the common stock of Solar Company for $320,000. Solar has common stock, other paid-in capital in excess of par, and retained earnings of $50,000,...
-
6 ES-15 (Preparation of a Statement of Cash Flows) Presented below is a condensed version of the cor (amounts in millions). parative statements of financial position for Yoon Corporation for the last...
-
During August 2014, Packer Manufacturing had the following cash receipts and disbursements: Cash received from customers .......... $319,000 Cash received from selling equipment ....... 11,200 Cash...
-
The protein lysozyme unfolds at a transition temperature of 75.5 C and the standard enthalpy of transition is 509 kJmol 1 . Calculate the entropy of unfolding of lysozyme at 25.0 C, given that the...
-
Calculate the change in entropy of the system when 10.0 g of ice at 10.0 C is converted into water vapour at 115.0 C and at a constant pressure of 1bar. The constant-pressure molar heat capacity of H...
-
The speed of sound increases slightly with temperature. (a) Does the fundamental frequency of the bottle increase, decrease, or stay the same as the air heats up on a warm day? Explain. (b) Find the...
-
What are the major immediate concerns for the HR manager in Austral Group SAA when merging two different organizational cultures - in this case, Peruvian and Norwegian cultures?
-
Explain the relation between the corporate, business and functional strategies. Please produce an in-depth explanation.
-
Consider the problem of terrorism during Radical Reconstruction. If you had been an adviser to the President, how would you propose to deal with the problem? Give a minimum of TWO examples and fully...
-
describe at least one element of an Airport Master Plan. Discuss the importance of this element and how it fits into the overall Airport Master Plan document to include its processes and objectives.
-
It is suggested that Wikipedia has replaced the hardback encyclopedia books, such Encyclopedia Brittanica. What other ways do you foresee technology changing businesses that have been around for...
-
When a cannon rigidly mounted on a large boat is fired, is momentum conserved? Explain, being careful to clearly define the system being considered.
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
Hexane and perfluorohexane show partial miscibility below 22.70C. The critical concentration at the upper critical temperature is x = 0.355, where x is the mole fraction of C 6 F 14 . At 22.0C the...
-
Some polymers can form liquid crystal mesophases with unusual physical properties. For example, liquid crystalline Kevlar (3) is strong enough to be the material of choice for bulletproof vests and...
-
Refer to the information in Exercise 6.15(b) and sketch the cooling curves for liquid mixtures in which x(B 2 H 6 ) is (a) 0.10, (b) 0.30, (c) 0.50, (d) 0.80, and (e) 0.95. Data in Exercise 6.15(b)...
-
Nelo Partnership had three partners, whose capital balances on June 30 were as follows: Jack $50,000, Andy $35,000, Nick $22,000. The profit-sharing ratio is 6:4:2 (Jack, Andy, Nick). On July 1,...
-
Alex buys a Blu-ray disc costing $14.49. Use the table below to find the sales tax on this item. Amount of Sale ($) Tax ($) 13.70 13.89 0.69 13.90 14.09 0.70 14.10 14.29 0.71 14.30 14.49 0.72 14.50...
-
Show partial income statements through gross margin for all three methods, assuming both products are further processed into Current Attempt in Progress It's mind - boggling the number of products...

Study smarter with the SolutionInn App


