In an experiment on the Pt|H 2 |H + electrode in dilute H 2 SO 4 the
Question:
In an experiment on the Pt|H2|H+ electrode in dilute H2SO4 the following current densities were observed at 25°C. Evaluate α and j0 for the electrode.
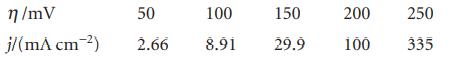
How would the current density at this electrode depend on the overpotential of the same set of magnitudes but of opposite sign?
Transcribed Image Text:
η/mV j/(m^ cm-²) 50 100 150 2.66 8.91 29.9 200 100 250 335
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 16% (6 reviews)
Solution Using the equations for PtH2H and PtH2H in dilute H2SO4 we have Now b...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In an experiment on the Pt IH,I H+ electrode in dilute H,S04 the following current densities were observed at 25e. Evaluate ET and jo for the electrode. How would the current density at this...
-
The following data were observed in an experiment on the photoelectric effect from potassium: Graphically evaluate these data to obtain values for the work function and Plancks constant. 1019 Kinetic...
-
In an experiment on soybean varieties, individually potted soybean plants were grown in a greenhouse, with 10 plants of each variety used in the experiment. From the harvest of each plant, five seeds...
-
Differentiate implicitly to find 2x + 5xy + 5y +16y - 8 = 0; dy dx . Then find the slope of the curve at the given point. (-2,0)
-
The data in the file named Fast100 were collected by D. L. Green & Associates, a regional investment management company that specializes in working with clients who wish to invest in smaller...
-
Evaluate the determinant. 5 -3 -2 -5 3 2.
-
Why do nations trade?.L01
-
During May, Joliet Fabrics Corporation manufactured 500 units of a special multilayer fabric with the trade name Stylex. The following information from the Stylex production department also pertains...
-
Hol Chong Transport, Ltd., operates a fleet of dellvery trucks in Singapore. The company has determined that if a truck is driven 162,000 kilometers during a year, the average operating cost is 13.2...
-
Tungsten Company, Inc., sells heavy construction equipment. There are 10,000 shares of capital stock outstanding. The annual fiscal period ends on December 31. The following condensed trial balance...
-
In an experiment on the adsorption of oxygen on tungsten it was found that the same volume of oxygen was desorbed in 27 min at 1856 K and 2.0 min at 1978 K. What is the activation energy of...
-
Nitrogen gas adsorbed on charcoal to the extent of 0.921 cm 3 g 1 at 490 kPa and 190 K, but at 250 K the same amount of adsorption was achieved only when the pressure was increased to 3.2 MPa. What...
-
In Problems 1-3, find the general solution to each differential equation. 1. dy/dt = 4.6e-0.05t 2. dy = (64 + 76x - 36x2) dx 3. dy/dx = 4x/y - 3
-
Propose how these mechanisms can be used to build a strategic business partnership, close the gap between management / leadership and employees while building a cohesive culture that adds value,...
-
1.What risks does the company face? 2. What is role for ERM at Swissgrid or most any company? 3. What risk management processes has Meyer installed at Swissgrid? Assess their strengths and...
-
Elizabeth's Country Wares How many workers does Elizabeth have and what does each of them do? What type of work does Elizabeth do for the CP product line? How long does it take to do the underglazing...
-
Do you support the policy of not allowing some Chinese nationals to attend graduate school in the United States because of national security concerns?
-
Using your product or service name or category, do a search using the following phrase: Find a (insert the name of your product or service here...) near me. For instance, using my Mobile Notary...
-
Evaluate lim t0 t 3 /tan 3 (2t).
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
One mole of H 2 O(l) is super cooled to 3.75C at 1 bar pressure. The freezing temperature of water at this pressure is 0.00C. The transformation H 2 O(l) H 2 O(s) is suddenly observed to occur. By...
-
An athlete at high performance inhales ~3.75 L of air at 1.0 atm and 298 K at a respiration rate of 32 breaths per minute. If the exhaled and inhaled air contain 15.3 and 20.9% by volume of oxygen,...
-
The temperature of 1.75 moles of an ideal gas increases from 10.2C to 48.6C as the gas is compressed adiabatically. Calculate q, w, U, and H for this process assuming that C V ,m = 3/2 R.
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


