Name the alkane: CHs CHy-CH-CH -HS CH2-CH
Question:
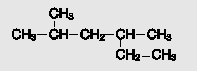
Transcribed Image Text:
CHs CHy-CH-CH ҫн-сHS CH2-CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (9 reviews)
2 4dimethylhexan...View the full answer

Answered By

Tamil Elakkiya Rajendran
I'm currently involved in the research in the field of Biothermodynamics, Metabolic pathway analysis and computational Biology. I always prefer to share my knowledge whatever I have learnt through my degree whenever time permits.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
All the parts of this problem refer to the alkane having the carbon skeleton shown. (a) What is the molecular formula of this alkane? (b) What is its IUPAC name? (c) How many methyl groups are...
-
Refer to Table 2.4 as needed to answer the following questions: (a) Octacosane has been found to be present in a certain fossil plant. Write a condensed structural formula for octacosane. (b) What is...
-
Name the class to which each of the compounds used in this experiment belongs (eg. alcohol, alkane, ester, etc.)
-
The AFL-CIO has undertaken a study of the yearly salaries (in thousands of dollars) of 30 administrative assistants. The organization wants to predict salaries from several other variables. The...
-
For each of the following overhead costs, suggest one method of apportioning cost to cost centre: (a) Employees' holiday pay; (b) Depreciation of floor-polishing machines used in all production...
-
Compare a display screen and a mobile device as output methods.
-
18. How can you determine whether or not a governmental fund should be considered major?
-
Consider a set of three periodic tasks with the execution profiles of Table. Develop scheduling diagrams similar to those of Figure for this set of tasks. Process Arrival Time Execution Time 10 10...
-
Selected hypothetical comparative statement data for the giant bookseller Barnes & Ne are as of the end of the fiscal year (in millions). 2022 2021 Net sales $5,600.0 $5,500.0 Cost of goods sold...
-
Refer to the following financial statements for Kodak: Liabilities and shareholders equity Current liabilities Required: Prepare forecasts of its income statement, balance sheet, and statement of...
-
A papyrus scroll was buried with King Tutankhamen 3330 years ago. The half-life of carbon-14 is 5730 years. The papyrus scroll sample had an initial decay of 15.3 disintegrations per minute per gram...
-
Name the alkene: CHs- CH-CH-- CH2-CHa
-
What is family life cycle? LO-3
-
Problem 228: The derivative is dz dt = = atb where a , and b =
-
Write a Python program which will take N names from the user. Create a dictionary from the N names that will hold First_name, Middle_name and Last_name in separate keys. The inputs will take N at...
-
2 Finding Poles and Zeros from a Bode Plot Consider the magnitude portion of the Bode plot in Figure 3. Based on the linear approxi- mation in red, find the transfer function G(s). 5 0 -5 10 -10 -15...
-
Indicate whether the following statements are "TRUE" or "FALSE" 1- Financial accounting is considered to be the backbone to top management. 2- Cost accounting identifies, summarizes and interprets...
-
Refer to case 3 shown above. Assume that Beta Division is now receiving an 3% price discount from the outside supplier. a. What is Alpha Division's lowest acceptable transfer price? b. What is Beta...
-
Write a power series that has the indicated interval of convergence. Explain your reasoning. [-3, 7]
-
For the following exercises, find the area of the triangle. Round to the nearest hundredth. 22 50 36
-
Ethylene glycol (antifreeze) has a density of 1.11 g/cm 3 . a. What is the mass in g of 417 mL of ethylene glycol? b. What is the volume in L of 4.1 kg of ethylene glycol?
-
A supposedly gold nugget displaces 19.3 mL of water and has a mass of 371 g. Could the nugget be made of gold?
-
Glycerol is a syrupy liquid often used in cosmetics and soaps. A 3.25 L sample of pure glycerol has a mass of 4.10 * 10 3 g. What is the density of glycerol in g/cm 3 ?
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


