Natural gas that contains methane, ethane, and propane is to be burned with humid air. The adiabatic
Question:
Natural gas that contains methane, ethane, and propane is to be burned with humid air. The adiabatic flame temperature is to be calculated from specified values of the following quantities:

(a) Without doing any calculations, predict the direction of change (increase, decrease, no change) in the adiabatic flame temperature you would expect for an increase in (i) yCH4 with yC3H8 held constant, (ii) Tf , (iii) Ta, (iv) Pxs, and (v) yW0. Briefly state your reasoning for each variable.
(b) For a basis of 1 g-mole of natural gas, calculate the gram-moles of each molecular species in the feed and product streams, assuming complete combustion and negligible CO formation. The answer should be expressed in terms of the variables given above.
(c) Given below are expressions for the specific enthalpies of the feed and product components, relative to their elements at 25°C.
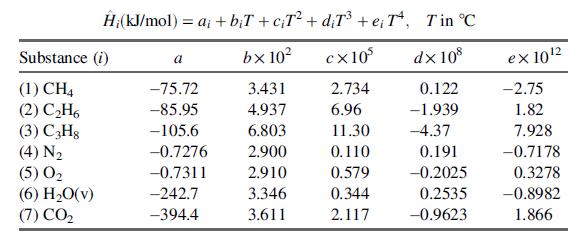
Derive the given expression for the specific enthalpy of methane from the heat capacity data in Table B.2. Then show that ΔH for the reactor is given by an expression of the form
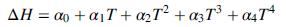
where T is the product temperature, and
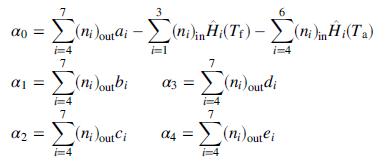
(d) Write a spreadsheet program to take as input values of yCH4 , yC3H8 , Tf , T|a, Pxs, and yW0, and to solve the energy balance equation [ΔH(T) = 0] to determine the adiabatic flame temperature. Run the program for the following sets of input variable values:
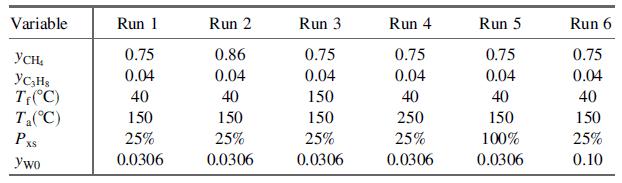
Suggestion: Near the top of the spreadsheet, enter the values of a, b, c, d, and e for each species. Starting several rows below the last of these entries, list in Column A labels for the input variables and all calculated variables (component molar flow rates, specific enthalpies, Tad, α0, α1, …, α4, ΔH) and enter in adjacent columns the corresponding values or formulas for these variables in successive runs. (Solution for Run 1: Tad = 1743:1°C.)
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





