Predict which of the bent molecules, BH 2 or NH 2 , should have the larger bond
Question:
Figure 24.11
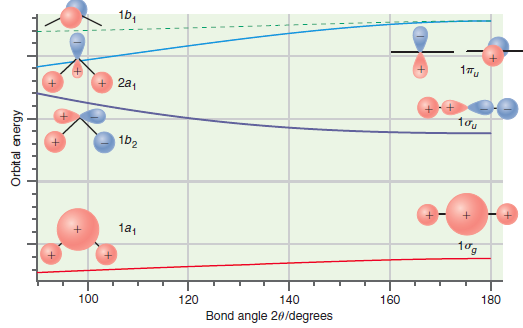
Transcribed Image Text:
16, 1Tu + 2a, 1b2 1a1 180 160 140 100 120 Bond angle 20/degrees Orbital energy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
Both molecules are equivalent through the 2a 1 1 orbital Howev...View the full answer

Answered By

Aijaz Khan
I am highly enthusiastic about tutoring. I share a friendly but professional relationship with my students. After completing my electrical engineering I actually taught a course to undergraduates for GATE exam as T.A and it was a brilliant experience and I was one among very few to finish my course on time. I have also helped professors to prepare lessons for my junior fellows while doing my undergrad. Every time I have taught so far, the response has been very heart warming. I hope to continue this and keep on improving it till I am here. Apart from this I have conducted many one on one tutoring lessons.
I believe in focussing on basic and core concepts inorder to keep students' interest alive. My only aim while tutoring is to make student understand concept in such a way that he/she can explain the learnt topic to anyone. Apart from this problem solving is my main focus while tutoring.
I hope to work with SolutionInn for long time. Hope my students will feel the difference.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict whether the ground state or the first excited state of CH 2 should have the larger bond angle on the basis of the Walsh correlation diagram shown in Figure 24.11. Explain your answer. 1b, 17u...
-
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown. (a) Draw a mechanism for the hydrolysis of this compound under...
-
Predict which of the following liquids has greater surface tension: ethanol (C2H5OH) or dimethyl ether (CH3OCH3)?
-
How well employees modify their thoughts and behavior to align with and support a new or changing environment is known as Multiple Choice proactive task performance. proficient task performance....
-
Find the exact values of the remaining trigonometric functions of satisfying the given conditions. 1. tan = 15/8, sin > 0 2. cos = 8/17, tan < 0
-
If x > 0 and 2x 2 - 4x = 30, what is the value of x?
-
Consider an investor with power utility and an infinite horizon. Assume the capital market line is constant, so we can write J(w) instead of J(x,w)for the value function. (a) Define = (1 )r (1 )2...
-
Consider the quarterly sales data for Worthington Health Club shown here (also available on the worksheet C11P9 in the OM4 Data Workbook): a. Develop a four-period moving average model and compute...
-
On October 1, Velec Co., a U.S. company, contracted to purchase foreign goods requiring payment in euros 1 month after their receipt at Velec's factory. Title to the goods passed on December 15. The...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Show that the water hybrid bonding orbitals given by a = 0.55 2Pz + 0.71 2 px -0.45 2 ps b = 0.55 2pz - 0.71 2px - 0.45 2s are orthogonal.
-
Derive two additional mutually orthogonal hybrid orbitals for the lone pairs on oxygen in H 2 O, each of which is orthogonal to Ï a and Ï b , by following these steps: a. Starting with the...
-
Use a search engine to find recent news about the Consumer Confidence Index. After studying the news, write a short summary of what the Consumer Confidence Index is trying to measure, including a...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z090 43. Z0.02 44. Z0.05
-
Case Study X Ltd. has 10 lakhs equity shares outstanding at the beginning of the accounting year 2016. The appropriate P/E ratio for the industry in which D Ltd. is 8.35. The earnings per share is...
-
Notation of 0 + Using the same survey described in Exercise 1, the probability of randomly selecting 50 speaking characters from movies and getting 40 females is expressed as 0+. Does 0+ indicate...
-
A simple random sample of 10 pages from a dictionary is obtained. The numbers of words defined on those pages are found, with the results n = 10, x = 66.4 words, s = 16.6 words. Given that this...
-
Question 3 58.5 Average global temperature 1880-2013 58.0 $ 57.5 57.0 56.5 1880 1900 1920 1940 1960 1980 2000 2020 Year The graph above indicates that global temperatures have Ovaried randomly over...
-
A manufacturers total cost is C(q) = 0.001q 3 0.05q 2 + 40q + 4,000dollars, where q is the number of units produced. a. Use marginal analysis to estimate the cost of producing the 251st unit. b....
-
Dr. Chan obtained a $15,000 demand loan at prime plus 1.5% on September 13 from the Bank of Montreal to purchase a new dental X-ray machine. Fixed payments of $700 will be deducted from the dentists...
-
Derive an expression for the time dependence of the degree of polymerization for a stepwise polymerization in which the reaction is acid catalysed by the -COOH acid functional group. The rate law is...
-
Calculate the average polymer length in a polymer produced by a chain mechanism in which termination occurs by a disproportionation reaction of the form M + M M + :M.
-
Calculate the ratio of the mean cube molar mass to the mean square molar mass in terms of (a) The fraction p, (b) The chain length.
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


