Predict whether the ground state or the first excited state of CH 2 should have the larger
Question:
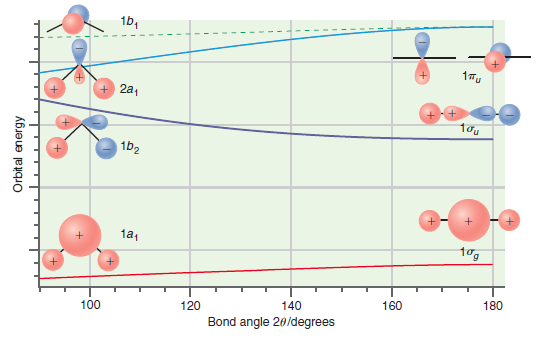
Transcribed Image Text:
1b, 17u 2a, 1b2 1a, tog 180 160 140 120 100 Bond angle 20/degrees Orbit al energy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
CH 2 has six valence electrons In the ground state the HOMO is the 2a ...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict which of the bent molecules, BH 2 or NH 2 , should have the larger bond angle on the basis of the Walsh correlation diagram in Figure 24.11. Explain your answer. Figure 24.11 16, 1Tu + 2a,...
-
Explain whether the electron arrangement for these atoms is the ground state or an excitedstate: a) Energy 3 Nitrogen b) Energy 2p 5 4 Carbon
-
Predict whether LiH + 2 and NH 2 should be linear or bent based on the Walsh correlation diagram in Figure 24.11. Explain your answers. Figure 24.11 1b, + 2a, 1b2 1a1 tog 100 120 140 160 180 Bond...
-
In an organization that has high employee satisfaction, ______. Multiple choice question. customer interactions are forced and scarce employee turnover is high more positive interactions take place...
-
Consider an angle in standard position with r = 12 centimeters, as shown in the figure. Describe he changes in the values of x, y, sin θ, cos θ, and tan θ as...
-
If the price of a sweater is marked down from $80 to $68, what is the percent discount? (Ignore the % symbol when gridding.)
-
Assume the investor has constant relative risk aversion . Define optimal consumption C and terminal wealth WT from the first-order conditions (14.7), and define Wt from (14.5). (a) Show that Wt = M1/...
-
Peter and Blair recently reviewed their future retirement income and expense projections. They hope to retire in 30 years and anticipate they will need funding for an additional 20 years. They...
-
During 2022, Eubanks Co. constructed various assets at a total cost of $20 million. The weighted average accumulated expenditures on assets qualifying for capitalization of interest during 2022 were...
-
Using the data in the student spreadsheet file Ethan Allen financials.XLSX ( to find the student spreadsheets for financial Analysis with Microsoft Excel, eighth edition, go to...
-
The occupied MOs of ammonia are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as localized or...
-
The occupied MOs of ethene are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as localized or...
-
If you are a scholar or researcher, try to classify all that you observe and know about the group that you are a member of into the basic categories of artifacts, espoused values, and basic...
-
Give a brief general description of the number of degrees of freedom. A. The number of degrees of freedom for a collection of sample data is the number of unique, non-repeated sample values. The...
-
Suppose you are given a data frame df. df = pd.DataFrame({'Click_ID':['A', 'B', 'C', 'D'], 'Count':[100, 200, 300, 400]}) In many data science projects, you are required to convert a dataframe into a...
-
Which of the following is an essential element of Six Sigma programs? Group of answer choices Setting specific goals for projects. Striving for low levels of Sigma. Striving for low levels of Cp and...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x=3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
The maximum employee earnings on which labour standards plan will be calculated on in 2019 was: Question 1 options: a) 67,500 b) 79,500 c) 86,500 d) 76,500 Question 2 (1 point) Question 2 options:...
-
In Exercises 9 through 22, find dy/dx by implicit differentiation. y 2 + 2xy 2 3x + 1 = 0
-
Ex. (17): the vector field F = x i-zj + yz k is defined over the volume of the cuboid given by 0x a,0 y b, 0zc, enclosing the surface S. Evaluate the surface integral ff, F. ds?
-
A second-order reaction of the type A + B P was carried out in a solution that was initially 0.050 mol dm 3 in A and 0.080 mol dm 3 in B. After 1.0 h the concentration of A had fallen to 0.020 mol...
-
Cyclopropane isomerizes into propene when heated to 500C in the gas phase. The extent of conversion for various initial pressures has been followed by gas chromatography by allowing the reaction to...
-
The rate constant for the first-order decomposition of N 2 O 5 in the reaction 2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g) is k = 3.38 10 5 s 1 at 25C. What is the half-life of N 2 O 5 ? What will be the...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


