The occupied MOs of ethene are shown next along with the MO energies. Indicate which AOs are
Question:
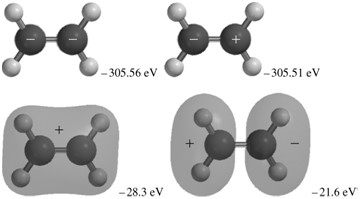

Transcribed Image Text:
+. -305.51 eV -305.56 eV -28.3 eV -21.6 eV -17.6 eV -16.1 ev -13.8 eV -10.3 eV
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
The MOs corresponding to the energies 30356 eV and 30351eV are the inphase and outofphase combinatio...View the full answer

Answered By

Mercy Kangai
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past seven years. I can bring value to your business and help solve your administrative assistant issues. I have extensive experience in marketing and small business management.
4.80+
27+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The occupied MOs of ammonia are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as localized or...
-
The occupied MOs of hydrogen cyanide are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as...
-
Indicate the type of solute-solvent interaction (Section 11.2) that should be most important in each of the following solutions: (a) CCl4 in benzene (C6H6), (b) Methanol (CH3OH) in water, (c) KBr in...
-
Juanita Torres is 35-years old and lately has been working with her financial planner. She is attempting develop a long-term savings and investment program. She has been thinking about all the major...
-
When f (t) = sin t and g (t) = cos t, is h (t) = f (t) g (t) even, odd, or neither? Explain.
-
At the beginning of the week, the ratio of cats to dogs at Glennas Pet Store was 4 to 5. By the end of the week, the number of cats had doubled, while the number of dogs had increased by 12. If the...
-
Assume aggregate consumption C and its expected growth rate satisfy dC C = dt + dB1 d = ( )dt + dB1 + 1 2 dB2 for constants , , , , and and independent Brownian motions B1 and B2. Then, the...
-
An engineering student bought a car at a local used car lot. Including tax and insurance, the total price was $3000. He is to pay for the car in 12 equal monthly payments, beginning with the first...
-
1. On January 1, 2022, Champion Inc. purchased a 10-year bond. Interest is payable semi- arks annually on January 1 and July 1 of each year. The bonds mature on January 1, 2032. Other information...
-
Which of the following statements is correct? a) The nested Turing machines can simulate other Turing machines. b) Boolean satisfiability problem is unsolvable. c) The Recursive enumerated language...
-
Predict whether the ground state or the first excited state of CH 2 should have the larger bond angle on the basis of the Walsh correlation diagram shown in Figure 24.11. Explain your answer. 1b, 17u...
-
Use the geometrical construction shown in Example Problem 24.8 to derive the electron MO levels for cyclobutadiene. What is the total energy of the molecule? How many unpaired electrons will the...
-
Compile a combined list of the 10 most common products the average college student might use.
-
Based on a survey, assume that 42% of consumers are comfortable having drones deliver their purchases. Suppose that we want to find the probability that when six consumers are randomly selected,...
-
What is the social location that determines this speech community? Is it determined by race, class, gender, sexuality, or some other social location? What makes this speech community unique? What are...
-
Write a program named SumOfNumberOfSquares.java that prompts user to enter a number of integers and calculates the sum of their squares. The following is a sample run. The green fonts represent user...
-
6.4 Charles Augustin de Coulomb was a French physicist who is best known for formulating the law that calculates the force between two electric charges. To honor Coulomb, the unit of electric charge...
-
What amount of cash payments to suppliers will be reported by Indigo Company for the year ended December 31, 2024?
-
In Exercises 13 through 24, compute the derivative of the given function and find the equation of the line that is tangent to its graph for the specified value x = c. f(x) = 2; c = 13
-
In the simple quantity theory of money, what will lead to an increase in aggregate demand? In monetarism, what will lead to an increase in aggregate demand?
-
At 518C, the half-life for the decomposition of a sample of gaseous acetaldehyde (ethanal) initially at 363 Torr was 410 s. When the pressure was 169 Torr, the half-life was 880 s. Determine the...
-
The second-order rate constant for the reaction is 0.11 dm 3 mol 1 s 1 . What is the concentration of ester after (a) 10 s, (b) 10 min when ethyl acetate is added to sodium hydroxide so that the...
-
Sucrose is readily hydrolysed to glucose and fructose in acidic solution. The hydrolysis is often monitored by measuring the angle of rotation of plane polarized light passing through the solution....
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


