The occupied MOs of ammonia are shown next along with the MO energies. Indicate which AOs are
Question:
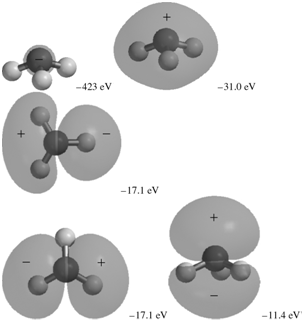
Transcribed Image Text:
-31.0 eV -423 eV -17.1 ev -17.1 eV -11.4 eV
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The MO with an energy of 423 eV corresponds to the 1s AO on N It is a loca...View the full answer

Answered By

Sumit kumar
I am an experienced online essay writer with a thorough understanding of any curriculum.and subject expert at Chegg for mathematics, CS subjects..
4.90+
5+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The occupied MOs of ethene are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as localized or...
-
The occupied MOs of hydrogen cyanide are shown next along with the MO energies. Indicate which AOs are most important in each MO and indicate the relative phases of the AOs. Classify the MOs as...
-
Indicate the type of solute-solvent interaction (Section 11.2) that should be most important in each of the following solutions: (a) CCl4 in benzene (C6H6), (b) Methanol (CH3OH) in water, (c) KBr in...
-
What is the is the effect of abolition of an import quota on (i) national saving, (ii) domestic investment, (iii) NCO, (iv) the real exchange rate, and (v) net exports?
-
(a) Use a graphing utility to complete the table. (b) Make a conjecture about the relationship between sin and sin(180 ). 0 20 0 40 60 80 sin e sin(180 0)
-
From 1970 to 1990, the percent increase in the U.S. consumption of nuclear energy was closest to A) 96% B) 240% C) 2,400% D) 3,400% 1950 1970 1990 2010 U.S. ENERGY CONSUMPTION (Quadrillion BTU...
-
Adopt the assumptions of Section
-
Consider the following annual returns of Estee Lauder and Lowes Companies: Compute each stocks average return, standard deviation, and coefficient of variation. Which stock appears better?Why? Year 1...
-
Franklin Products Limited manufactures and distributes a number of products to retailers. One of these products, SuperStick, requires four kilograms of material D236 in the manufacture of each unit....
-
A study was done on a diesel-powered light-duty pickup truck to see if humidity, air temperature, and barometric pressure influence emission of nitrous oxide (in ppm). Emission measurements were...
-
Use the VSEPR method to predict the structures of the following: a. PF 3 b. CO 2 c. BrF 5 d. SO 2 3
-
Predict whether the ground state or the first excited state of CH 2 should have the larger bond angle on the basis of the Walsh correlation diagram shown in Figure 24.11. Explain your answer. 1b, 17u...
-
How is cash held as a compensating balance reported on the balance sheet?
-
Dr. Stanley and his staff are attempting to utilize effective ways to both increase the revenue for the practice and allow patients to schedule visits without a lengthy delay. Which of the following...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Suppose the total cost in dollars of manufacturing q units is C(q) = 3q 2 + q + 500. a. Use marginal analysis to estimate the cost of manufacturing the 41st unit. b. Compute the actual cost of...
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
The reaction mechanism involves an intermediate A. Deduce the rate law for the reaction. AA+A (fast) A+B P (slow)
-
A reaction 2 A P has a second-order rate law with k = 3.50 10 4 dm 3 mol 1 s 1 . Calculate the time required for the concentration of A to change from 0.260 mol dm 3 to 0.011 mol dm 3 .
-
Consider the dimerization 2 A A 2 , with forward rate constant k a and backward rate constant k b . (a) Derive the following expression for the relaxation time in terms of the total concentration of...
-
Justice Corporation Comparative Balance Sheet December 31, 2025 and 2024 2025 2024 Assets Current Assets: $ Cash and Cash Equivalents 2,254 $ 1,876 Justice Corporation reported the following...
-
The Fields Company has two manufacturing departments forming and painting. The company uses the FIFO method of process costing at the beginning of the month the forming department has 33.000 units in...
-
A comparative balance sheet for Lomax Company containing data for the last two years is as follows: Lomax Company Comparative Balance Sheet This Year Last Year $ 96,000 $ 70,000 640,000 672,500...

Study smarter with the SolutionInn App


