Show that the osmotic pressure of a real solution is given by V=RT ln aA. Go on
Question:
Show that the osmotic pressure of a real solution is given by ΠV=–RT ln aA. Go on to show that, provided the concentration of the solution is low, this expression takes the form ΠV=ϕRT[B] and hence that the osmotic coefficient ϕ (which is defined in Problem 5E.2) may be determined from osmometry.
Data in Problem 5E.2
The osmotic coefficient ϕ is defined as ϕ=−(xA/xB) ln aA. By writing r=xB/xA, and using the Gibbs–Duhem equation, show that we can calculate the activity of B from the activities of A over a composition range by using the formula
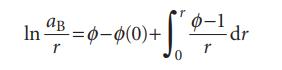
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:





