Question: At sufficiently high temperatures, the van der Waals equation has the form P L RT>(V m - b). Note that the attractive part of the
At sufficiently high temperatures, the van der Waals equation has the form P ‰ˆ L RT>(Vm- b). Note that the attractive part of the potential has no influence in this expression. Justify this behavior using the potential energy diagram of Figure 1.10.
Figure 1.10
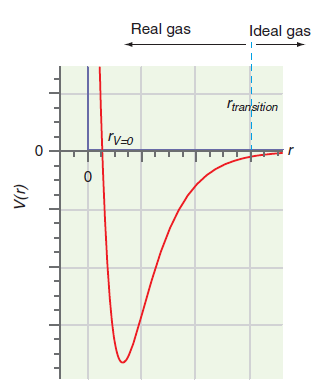
Ideal gas Real gas Itrarlsition (1)A
Step by Step Solution
★★★★★
3.27 Rating (179 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

At high temperatures the energy of the molecule is lar... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


