The parameter a in the van der Waals equation is greater for H 2 O than for
Question:
Figure 1.10
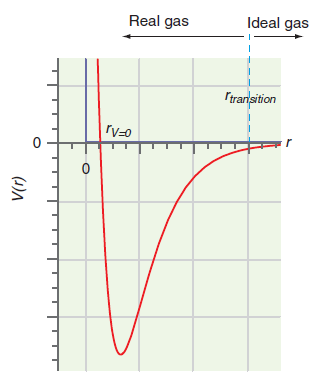
Transcribed Image Text:
Ideal gas Real gas Itransition ľV=0 (1)A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
It says that the ...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the van der Waals equation, why is a term added to the observed pressure and why is a term subtracted from the container volume to correct for nonideal gas behavior?
-
Show that the van der Waals equation can be written as a cubic equation in the compressibility factor involving the reduced pressure and reduced temperature as 27 P 2 512T 3
-
Deviations from the Ideal-Gas Equation For carbon dioxide gas (CO2), the constants in the van der Waals equation are a = 0.364 j m3/mol2 and b = 4.27 X 10-5 m3/mol (a) If 1.00 mol of CO2 gas at 350 K...
-
Compute the Regular Pay, Overtime Rate, Overtime Pay, and Gross Pay for each employee. Employees are paid weekly. Filing Employee Status Henry Smith Parker Liam Melanie Total S S S M S Hours Pay...
-
1. Read the article sent to you via e-mail "Framing the Impact of Culture on Health: A Systematic Review of the PEN-3 Cultural Model and its Application in Public Health Research and Interventions"....
-
Set up appropriate systems of two linear equations and solve the systems algebraically. All data are accurate to at least two significant digits. A small isolated farm uses a windmill and a gas...
-
Under USHA,_____ refers to grouping revenue and expense items, so that the performance of the individual responsible for that particular segment of the operation can be measured and judged.
-
Richard Penn lives in Harrisburg, Pennsylvania. Richard is the president of an architectural firm. Richard has become known throughout the community for excellent work and honesty in his business...
-
Question 6 Not yet answered Marked out of 0.50 A company that uses a job order costing system would make the following entry to record the flow of direct materials into production: Select one: a....
-
1. How do information technologies contribute to the business success of Sew What? Inc.? Give several examples from the case regarding the business value of information technology that demonstrate...
-
Compound A and compound B are constitutional isomers with molecular formula C 3 H 7 Cl. When compound A is treated with sodium methoxide, a substitution reaction predominates. When compound B is...
-
Compound A and compound B are constitutional isomers with molecular formula C 4 H 9 Cl. Treatment of compound A with sodium methoxide gives trans-2-butene as the major product, while treatment of...
-
Refer to the income statements of Dell Inc. included in the company's financial statements in Appendix B at the back of the text. Required: 1. What was the percentage increase or decrease in the...
-
(AVR) PR=IAVR=1R = (power dissipated by a resistor) (28.12) R
-
As a manager and an entrepreneur, you will face a new challenge - business venture structured on the theory of the firm. You are opening a restaurant in your selected town in the State of NY (please...
-
Install on ubuntu , please provide a screenshot for each step 1)How to install base64 on ubuntu 2)What kind of analysis is performed by Cuckoo? How to install Cuckoo on ubuntu?
-
rt a letter to Rose McBride. Writing Plan - Refusal to a Request Rubric Buffer: Start with a neutral statement on which both reader and writer can agree, such as a compliment, appreciation, a quick...
-
FACTS: The Budvar Company sells parts to a foreign customer on December 1, Year 1, with payment of 20,000 crowns to be received on March 1, Year 2. Budvar enters into a forward contract (with a...
-
Factor out the greatest common factor. 5(m + p) - 10(m + p) - 15(m + p)4
-
In order to get an idea on current buying trends, a real estate agent collects data on 10 recent house sales in the area. Specifically, she notes the number of bedrooms in each house as follows: a....
-
When 178J of energy is supplied as heat to 1.9 mol of gas molecules, the temperature of the sample increases by 1.78 K. Calculate the molar heat capacities at constant volume and constant pressure of...
-
When 2.0 mol CO2 is heated at a constant pressure of 1.25 atm, its temperature increases from 250 K to 277 K. Given that the molar heat capacity of CO2 at constant pressure is 37.11 J K-1 mol-1,...
-
A sample of 5.0 mol CO2 is originally confined in 15 dm ' at 280 K and then undergoes adiabatic expansion against a constant pressure of 78.5 kPa until the volume has increased by a factor of 4.0....
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


