The Clapeyron equation does not apply to second-order phase transitions, but there are two analogous equations, the
Question:
The Clapeyron equation does not apply to second-order phase transitions, but there are two analogous equations, the Ehrenfest equations, that do. They are:
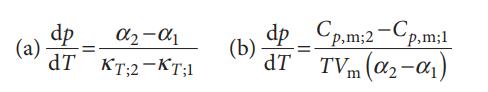
Where α is the expansion coefficient, κT the isothermal compressibility, and the subscripts 1 and 2 refer to two different phases. Derive these two equations. Why does the Clapeyron equation not apply to second-order transitions?
Transcribed Image Text:
α₂-α1 TKT;2-KT;1 dp (a) = dp_Cp,m;2-Cp,m;1 (b) dp= dT TVm (0₂-01)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Answer The Ehrenfest equations can be derived by taking the partial derivatives of the Gibbs free en...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The law of demand does not apply to professional base-ball players. Since each team already has the maximum number of players allowed on its squad, a reduction in the wage rate that must be paid for...
-
Generally, the BFOQ defense does not apply to customer preference. But recently, some clients have been pressuring their law firms to staff their cases with female and minority lawyers. If a firm...
-
Which of the following properties does not apply to multiplication of matrices? A. Commutative B. Associative C. Distributive D. Identity
-
The Justice Department has been asked to review a merger request for a market with the following four firms. Firm Assets A .......... $156 million B .......... 130 million C .......... 45 million D...
-
ABC Toy Company earned $ 357 million of net income in 2015 and paid $ 45 million in dividends. It issued no new stock. Complete the Stockholders' Equity section for ABC Toy Company: December 31 (in...
-
High Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
Baiting traps to maximize beetle catch. A field experiment was conducted to compare the effectiveness of different traps for catching beetles (Journal of Chemical Ecology, Vol. 94, 2011). Paraffin...
-
The U.S. Cable Company uses a distribution system with five distribution centers and eight customer zones. Each customer zone is assigned a sole source supplier; each customer zone receives all of...
-
On October 1, 2019, Santana Rey launched a computer services company, Business Solutions, that is organized as a corporation and provides consulting services, computer system installations, and...
-
Sulfide ion was determined by indirect titration with EDTA. To a solution containing 25.00 mL of 0.04332 M Cu(ClO4)2 plus 15 mL of 1M acetate buffer (pH 4.5) were added 25.00 mL of unknown sulfide...
-
Find the stationary distribution for the Ehrenfest Markov Chain. Data from in stationary distribution If we have an ergodic Markov Chain, we know that each state will be visited infinitely often....
-
Combine the barometric formula, p=p 0 e a/H , where H=8 km, for the dependence of the pressure on altitude, a, with the ClausiusClapeyron equation, and predict how the boiling temperature of a liquid...
-
When a metal is heated its density decreases. There are two sources that give rise to this decrease of : (1) the thermal expansion of the solid and (2) the formation of vacancies (Section 4.2)....
-
Small paragraphs address these sub questions, 1. What is governance? 2. What drives the strategy orientation of the leadership ? 3. How do strategies trickle down to management and translate to...
-
Accommodating guests with disabilities must be a priority for venue and event managers. With a growing population of persons with disabilities, it's important to identify current trends on how venues...
-
a. (6) The Fibonacci sequence is defined by Fo= 0, F = 1, and, for all n > 2, Fn = Fn-1+ Fn-2- Prove that Fn O(2"), without using part (b). b. (6) Let An C {0, 1}" be the set of binary strings of...
-
Customer purchase history matrix. A store keeps track of its sales of products from K different product categories to N customers over some time period, like one month. (While it doesn't matter for...
-
2. A Beautiful Circuit Answer the following questions about the 4-resistor circuit shown below. A. Calculate the equivalent resistance of this circuit. B. Calculate the power delivered to the circuit...
-
Two forces are applied to a merry-go-round with a radius of 1.2 m as shown in the diagram below. One force has a magnitude of 80 N and the other a magnitude of 50 N. a. What is the torque about the...
-
Write a paper detailing a geographic information system (GIS) of your own design that would utilize data in an original manner.
-
A sample consisting of 1.00 mol of a van der Waals gas is compressed from 20.0 dm 3 to 10.0 dm 3 at 300 K. In the process, 20.2 kJ of work is done on the gas. Given that = {(2a/RT) b}/C p,m, with C...
-
The standard enthalpy of combustion of cyclopropane is 2091 kJ mol 1 at 25C. From this information and enthalpy of formation data for CO 2 (g) and H 2 O(g), calculate the enthalpy of formation of...
-
The standard enthalpy of formation of ethylbenzene is 12.5 kJ mol 1 . Calculate its standard enthalpy of combustion.
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...

Study smarter with the SolutionInn App


