The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature.
Question:
The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature. By how much does the standard molar enthalpy of SO2(g) increase when the temperature is raised from 298.15K to 1500K?
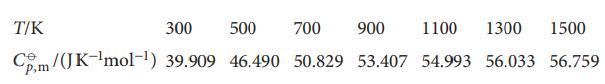
Transcribed Image Text:
T/K 300 500 700 900 1100 1300 1500 Cm/(JK-¹mol-¹) 39.909 46.490 50.829 53.407 54.993 56.033 56.759
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
Answer and thorough explanation The standard molar enthalpy of a substance is given by H Hf CpT Tf w...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The following data show how the marginal external benefit and marginal private benefit associated with a soil treatment agent to control Japanese beetles vary with the gallons of the control agent...
-
The following data show the exam grades for two different sections of a statistics class that I teach. The first group of data is from a day section of traditional students. The second group of data...
-
The following data show the daily closing prices (in dollars per share) for IBM for November 3, 2005, through December 1, 2005 (Compustat, February 26, 2006). a. Define the independent variable...
-
As manager of a local pizza parlor, you want to develop a balanced scorecard so you can more effectively monitor the restaurants performance. Required a. Propose at least two goals for each...
-
Using the information from BE14- 24, prepare the journal entry to record the bond issue, assuming that Lee Equipment Company is an IFRS reporter. BE14-24 Lee Equipment Company issued 200 eight- year,...
-
A uniform distribution is defined over the interval from 6 to 10. a. What are the values for a and b? b. What is the mean of this uniform distribution? c. What is the standard deviation? d. Show that...
-
Describe how the following entities can influence public managers and public organizations. What formal and informal authority do they have that enables them to exert such influence? a. Public...
-
Carraway Seed Company Inc. has for many years cultivated and sold what are known as heritage plants and seeds. For example, the company has sought out older varieties of tomato plants that are no...
-
Credit Limit. Mary and Marty are interested in obtaining a home equity loan. They purchased their house five years ago for $, and it now has a market value of $. Originally, Mary and Marty paid $...
-
Cronbach's alpha is the most common measure of internal consistency (reliability") for measures. It is frequently used when we have several Likert scale questions from a survey, and we wish to...
-
Describe two calorimetric methods for the determination of enthalpy changes that accompany chemical processes.
-
A sample consisting of 1.00mol Ar is expanded isothermally at 20 C from 10.0dm 3 to 30.0dm 3 (i) Reversibly, (ii) Against a constant external pressure equal to the final pressure of the gas, (iii)...
-
Think about situations where your values have been in conflict. How have you resolved those conflicts? Now that you have studied the ethical decision-making frameworks in this chapter, what should...
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
How does nuclear fusion differ from nuclear fission? Explain.
-
On August 31, 2012, the balances of the accounts appearing in the ledger of Wood Interiors Company, a furniture wholesaler, are as follows:Prepare the August 31, 2012, closing entries for Wood...
-
Calculate the percentage difference in the fundamental vibration wavenumber of 23 Na 35 Cl and 23 Na 37 Cl on the assumption that their force constants are the same.
-
At low resolution, the strongest absorption band in the infrared absorption spectrum of 12 C 16 O is centred at 2150 cm 1 . Upon closer examination at higher resolution, this band is observed to be...
-
An object of mass 1.0 kg suspended from the end of a rubber band has a vibrational frequency of 2.0 Hz. Calculate the force constant of the rubber band.
-
The following amounts were reported on the December 31, 2022, balance sheet: Cash $ 8,000 Land 20,000 Accounts payable 15,000 Bonds payable 120,000 Merchandise inventory 30,000 Retained earnings...
-
Sandhill Co. issued $ 600,000, 10-year, 8% bonds at 105. 1.Prepare the journal entry to record the sale of these bonds on January 1, 2017. (Credit account titles are automatically indented when the...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.

Study smarter with the SolutionInn App


