The gas-phase decomposition of acetic acid at 1189 K proceeds by way of two parallel reactions: What
Question:
The gas-phase decomposition of acetic acid at 1189 K proceeds by way of two parallel reactions:
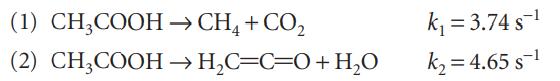
What is the maximum percentage yield of the ketene CH2CO obtainable at this temperature?
Transcribed Image Text:
(1) CH3COOH (2) CH3COOH → CH4 + CO₂ → H₂C=C=O + H₂O k₁ = 3.74 s ¹ k₂=4.65 s-¹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)

Answered By

Anurag Agrawal
I am a highly enthusiastic person who likes to explain concepts in simplified language. Be it in my job role as a manager of 4 people or when I used to take classes for specially able kids at our university. I did this continuously for 3 years and my god, that was so fulfilling. Sometimes I've skipped my own classes just to teach these kids and help them get their fair share of opportunities, which they would have missed out on. This was the key driver for me during that time. But since I've joined my job I wasn't able to make time for my passion of teaching due to hectic schedules. But now I've made a commitment to teach for at least an hour a day.
I am highly proficient in school level math and science and reasonably good for college level. In addition to this I am especially interested in courses related to finance and economics. In quest to learn I recently gave the CFA level 1 in Dec 19, hopefully I'll clear it. Finger's crossed :)
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Estimate the liquid diffusivity of acetic acid at 25oC in a dilute solution of:(a) Benzene,(b) Acetone,(c) Ethyl acetate, and(d) Water by an appropriate method. Compare the estimated values with the...
-
Find the viscosity of Acetic acid at 300K.
-
Calculate the volume of 47 g of acetic acid at 20 C ( = 1.05 g / mL).
-
How do recruitment and selection practices contribute to high performance in an organization?
-
PricewaterhouseCoopers Saratoga, in its 2005/2006 Human Capital Index Report, indicated the average number of days it took for an American company to fill a job vacancy in 2004 was 48 days. Sample...
-
Use a double integral to find the area of the region. One loop of the rose r = cos 3
-
2 Suponga que el presidente de una fbrica de alfombras le ha pedido que investigue la posibilidad de eliminar a los mayoristas de la compaa (quienes venden los productos a tiendas de alfombras,...
-
Preparing lessors journal entries for an operating lease and a capital lease. Sun Microsystems manufacturers an engineering workstation for $7,200 and sells it for $12,000. Although the workstation...
-
Part 1 of 3 Ayers, Inc has the following cost data for Product X and unit product cout using absorption conting when production is 250 units, 500 units and 2.500 unts (Click on the icon to view the...
-
Suppose that the California legislature passes a law that severely restricts carbon dioxide emissions from automobiles in that state. A group of automobile manufacturers files suit against the state...
-
A first-order decomposition reaction is observed to have the following rate constants at the indicated temperatures. Estimate the activation energy. k/(10-s-) /C 2.46 0 45.1 20.0 576 40.0
-
The following data have been obtained for the decomposition of N 2 O 5 (g) at 67C according to the reaction 2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g). Determine the order of the reaction, the rate...
-
Is there a relationship between corporate governance and social responsibility?
-
At 3 1 st March, 2 0 2 3 , AB Ltd , had an Authorized Capital of K 3 5 , 0 0 0 divided into 1 0 , 0 0 0 7 . 5 % noncumulative per share being due on 3 0 th June, 1 9 6 4 . per share paid, the...
-
A Leadership and Workforce Development Perspective. The literature review should discuss the related literature, organized by topic or themes (not a list of sources). A literature review includes...
-
Critical Success Factors (CSF) are elements that are necessary for an organization or a project to attain its objectives. For example, Chief Executive support is a CSF for corporate sustainability...
-
Ultra Ceramic Products presented the following data for its operations for the month of October, 2020: Dept 1 Work in process, July t. 1(Conversion costs, 60%) 7,000 units Transferred to Dept 2 Work...
-
Choose a global organizational leader who demonstrated how a high level of ethical communication via social media technologies have worked best at building trust with virtual stakeholders. Identify a...
-
Find the numbers at which f is discontinuous. At which of these numbers is f continuous from the right, from the left, or neither? Sketch the graph of f. 2 if x <1 f(x) = {3 x if 1 4
-
Which one of the following anhydrous chloride is not obtained on direct heating of its hydrated chloride? (A) BaCl2 (B) CaClz (C) MgCl2 (D) SrCl2
-
On the basis of the following proposed mechanism, account for the experimental fact that the rate law for the decomposition 2 N2O5 (g) → 4 NO2 (g) + O2 (g) is v=k [N205]' k,ki (I) NO NO.+NO, (2)...
-
Consider the following mechanism for the thermal decomposition ofR2: Where R2, PA' PB are stable hydrocarbons and Rand R' are radicals. Find the dependence of the rate of decomposition of R, on the...
-
Refer to Fig. 23.3 and determine the pressure range for a branching chain explosion in the hydrogen-oxygen reaction at (a) 700 K, (b) 900 K.
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...
-
Apple inc. CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (In milions, except number of shares which are reflected in thousands and par value) LABILITES AND SHAREHOLDERS' EQUITY: Current...

Study smarter with the SolutionInn App


