The molar heat capacity C P,m of SO 2 (g) is described by the following equation over
Question:
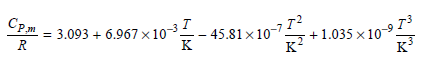
In this equation, T is the absolute temperature in kelvin. The ratios T€/K€ ensure that CP,m has the correct dimension. Assuming ideal gas behavior, calculate q, w, ΔU, and ΔH if 1.50 moles of SO2(g) is heated from 22.5°C to 1140.°C at a constant pressure of 1 bar. Explain the sign of w.
Transcribed Image Text:
Сри 3.093 + 6.967 x103÷- 45.81x10-7 -2 +1.035 ×10 K- 9. K K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
858 10 4 J U H PV H nRT 858 10 4 J 150 mol 8314 J mol 1 K 1 141315 K 29565 K 718 10 4 J W U ...View the full answer

Answered By

Muhammad Ahtsham Shabbir
I am a professional freelance writer with more than 7 years’ experience in academic writing. I have a Bachelor`s Degree in Commerce and Master's Degree in Computer Science. I can provide my services in various subjects.
I have professional excellent skills in Microsoft ® Office packages such as Microsoft ® Word, Microsoft ® Excel, and Microsoft ® PowerPoint. Moreover, I have excellent research skills and outstanding analytical and critical thinking skills; a combination that I apply in every paper I handle.
I am conversant with the various citation styles, among them; APA, MLA, Chicago, Havard, and AMA. I also strive to deliver the best to my clients and in a timely manner.My work is always 100% original. I honestly understand the concern of plagiarism and its consequences. As such, I ensure that I check the assignment for any plagiarism before submission.
4.80+
392+ Reviews
587+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In a certain polytropic process the volume of argon was in- creased a- 4.0 times. Simultaneously, the pressure decreased = 8.0 times. Find the molar heat capacity of argon in this process, assuming...
-
The molar heat capacity of a liquid is normally greater than its vapor-phase heat capacity at modest pressure and the same temperature. Why?
-
The heat capacity at constant volume of hydrogen sulfide at low pressures is C v [kJ/ (mol??C)] = 0.0252 + 1.547 x 10 ?5? T ? 3.012 X 10 ?9 T 2? where T is in ?C. A quantity of H 2 S is kept in a...
-
Graph the function y = (x + |x|). At what values of x does the derivative exist and what is the value of the derivative when it does exist?
-
When is the "Income Summary" account used? Assume there is a net loss for the period. Make all journal entries affecting Income Summary. You do not need to include numbers or specific account titles...
-
Find the required ratios. The electric current in a given circuit is the ratio of the voltage to the resistance. What is the current (1 V/1 = 1 A) for a circuit where the voltage is 24.0 mV and the...
-
Which growth strategies have been pursued by Starbucks and Dunkin Donuts in the past? Which strategies do you believe will be most successful for the two firms in the future? Why? THE COFFEE WARS In...
-
On January 1, 2007 Lani Company entered into a non-cancelable lease for a machine to be used in its manufacturing operations. The lease transfers ownership of the machine to Lani by the end of the...
-
Innovative Tech Incorporated (ITI) has been using the percentage of credit sales method to estimate bad debts. During November. ITI sold services on account for $150,000 and estimated that 1/4 of 1...
-
Recent graduate and world traveler Alastair Bor is planning a European trip. He is influenced by his curiosity about urban culture in the EU and by his study of international relations while he was...
-
Predict the geometry of each atom except hydrogen in the compounds below: a. b. c. d. - - I0 I0 :0I
-
Use the relation (U/V ) T = T(P/T) V P and the cyclic rule to obtain an expression for the internal pressure, (0U/0V )T , in terms of P, , T, and .
-
A spherical shell of radius R is divided into three conducting segments by two very thin air gaps located at latitudes 0 and 0 . The center segment is grounded. The upper and lower segments are...
-
Are investors too impatient in wanting returns from these investments? "Would Nordstrom be better off if it were owned by private investors, rather than as a publicly traded firm since this would...
-
how AI is used to discover new material, will it change the whole science environment?
-
QUESTION 1 Which one of the following statements is not part of the "bundle of rights" enjoyed by a fee simple owner of property? a. The right to possess the property b. The right to control what...
-
In order to pay his rent, Tom, a college student, has taken a job in the computer department of a local department store. His only responsibility is to answer telephone calls to the department, most...
-
How does database normalization impact data integrity and query performance in complex relational schemas ? Explain
-
In Exercises 5970, the domain of each piecewise function is (- , ).a. Graph each function.b. Use your graph to determine the functions range. f(x) x 2x 1 - if if x < 1 x 1
-
In Problem use absolute value on a graphing calculator to find the area between the curve and the x axis over the given interval. Find answers to two decimal places. y = x 3 ln x; 0.1 x 3.1
-
The equilibrium A ( B is first -order in both directions. Derive an expression for the concentration of A as a function of time when the initial molar concentrations of A and Bare [A]0 and [B]0. What...
-
Derive the integrated form of a third-order rate law v = k[A f [B] in which the stoichiometry is 2 A + B ( P and the reactants are initially present in (a) Their stoichiometric proportions, (b) With...
-
Show that the ratio t1/2/t3/4 where 1112 is the half-life and 13/4is the time for the concentration of A to decrease to t of its initial value (implying that t3/4 < t1/2) can be written as a function...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


