The partial pressures of Br 2 above a solution containing CCl 4 as the solvent at 25°C
Question:
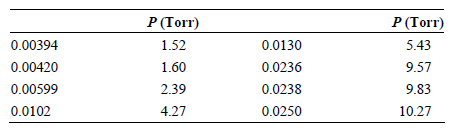
Transcribed Image Text:
P (Torr) 1.52 P (Torr) 5.43 9.57 9.83 0.00394 0.00420 0.0130 0.0236 0.0238 0.0250 1.60 2.39 0.00599 0.0102 4.27 10.27
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
The best fit line in the plot is PBr ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Your task in this problem will be to use a spreadsheet to generate a Txy diagram for a two-component system, using Raoult?s law to express the vapor?liquid equilibrium distribution of each species....
-
A 1.00- L gas sample at 100oC and 600. torr contains 50.0% helium and 50.0% xenon by mass. What are the partial pressures of the individual gases?
-
The partial pressures of an equilibrium mixture of N2O4(g) and NO2(g) are PN2O4 = 0.34 atm and PNO2 = 1.20 atm at a certain temperature. The volume of the container is doubled. Calculate the partial...
-
Crystal Cleaners dry cleans industrial clothing. The following excerpt from its PPE Subledger shows the component details regarding the dry cleaning equipment: Calculate depreciation on the dry...
-
Consider the following modifications to the Good and Rich Candy Company example from class. How would the aggregate planning strategy change in each case provided? Data from class lecture include:...
-
Suppose the economy is experiencing a recessionary gap. a. Draw a graph showing aggregate demand, short-run aggregate supply, and long-run aggregate supply. Label both axes, all curves, and the...
-
Understand international marketing communications. LO.1
-
Walmart Stores, Inc. (Walmart) is the largest retailing firm in the world. Building on a base of discount stores, Walmart has expanded into warehouse clubs and Supercenters, which sell traditional...
-
Perform a fundamental analysis evaluation for Armanino foods of distinction stock including important components as stock beta, companys price-to earnings ratio, earnings per share, book value and...
-
Which of the graphs in Fig. Q25.12 best illustrates the current I in a real resistor as a function of the potential difference V across it? Explain. Figure Q25.12 (a) (b) (c) (d)
-
A and B form an ideal solution. At a total pressure of 0.720 bar, y A = 0.510 and x A = 0.420. Using this information, calculate the vapor pressure of pure A and of pure B.
-
The data from Problem P9.20 can be expressed in terms of the molality rather than the mole fraction of Br2 . Use the data from the following table and a graphical method to determine the Henrys law...
-
Guess the value of the limit by evaluating the function f(x) = x 2 /2 x for x 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 50, and 100. Then use a graph of f to support your guess. lim 00-x 2*
-
Using the ideas of kinetic particle theory when you come home from school and open the door you can smell food being cooked
-
The following information relates to Salamat Corporation for the last year.Salamat uses direct labor hours as an overhead base. Estimated direct labor hours 360,000 hours Estimated manufacturing...
-
Code in matlab the translational motion via numeric integration of the orbit (two-body orbit sufficient). Use the orbital characteristics of the Centaur V upper stage from the Atlas V launch on...
-
Lolita Company has the following information available for June 2020: Beginning Work in Process Inventory (25% as to conversion) 20,000 units Started 130,000 units Ending Work in Process Inventory...
-
Question 3 (15 marks) Sporty Ltd. produces scooters and skateboards. At the beginning of the year, the following volume of activities were budgeted for the year: Production volume/units Direct labour...
-
If a car is moving at 50 miles per hour on a level highway, then its braking distance depends on the road conditions. This distance in feet can be computed by D(x) = 250/30x where x is the...
-
Why is inventory management important for merchandising and manufacturing firms and what are the main tradeoffs for firms in managing their inventory?
-
Calculate the standard Gibbs energy of the reaction 4 HCl(g) + O2(g) 2 Cl 2 (g) + 2 H 2 O(l) at 298 K, from the standard entropies and enthalpies of formation given in the Data section.
-
Suggest a physical interpretation of the dependence of the Gibbs energy on the temperature.
-
The enthalpy of vaporization of chloroform (CHCl 3 ) is 29.4 kJ mol 1 at its normal boiling point of 334.88 K. Calculate (a) The entropy of vaporization of chloroform at this temperature and (b) The...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


