The standard potential of the AgCl/Ag,Cl couple has been measured very carefully over a range of
Question:
The standard potential of the AgCl/Ag,Cl− couple has been measured very carefully over a range of temperature (R.G. Bates and V.E. Bowers, J. Res. Nat. Bur. Stand. 53, 283 (1954)) and the results were found to fit the expression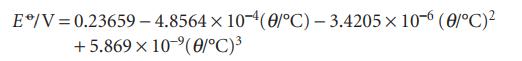
Calculate the standard Gibbs energy and enthalpy of formation of Cl−(aq) and its entropy at 298 K.
Transcribed Image Text:
E/V=0.23659-4.8564 × 10-4 (0/°C) -3.4205 x 10-6 (0/°C)² +5.869 x 10 ⁹(0/°C)³
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Standard potential of the AgClAgCl couple as per the fit expression E o V 023659 48564 x 10 4 x 298 ...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard potential of the Zn2+/ Zn electrode is -0.76 V at 25e. The exchange current density for H+ discharge at platinum is 0.79 mA cm-2 Can zinc be plated on to platinum at that temperature?...
-
The standard enthalpy of formation of H2O(l) at 298 K is 285.8 kJ/ mol. Calculate the change in internal energy for the following process at 298 K and 1 atm: H2O(l) H2(g) + O2(g) Eo = ?
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
A rectangular loop of wire 24 cm by 72 cm is bent into an L shape, as shown in FIGURE 23-49. The magnetic field in the vicinity of the loop has a magnitude of 0.035 T and points in a direction...
-
Consider the following set of observations: You should not assume these data come from a normal distribution. Test the hypothesis that these data come from a distribution with a median equal to 4....
-
Graph each function. Determine the largest open intervals of the domain over which each function is (a) increasing or (b) decreasing. (x) = (x - 1) 4 + 2
-
A typical exposure from a dental X-ray is 10 mrem. (See Table 30.4.) (a) Approximately how much energy is deposited to your head when you get a dental X-ray? (b) An object of mass 1 kg falls on your...
-
New Millennium Company earned $2.5 million in net income last year. It took depreciation deductions of $300,000 and made new investments in working capital and fixed assets of $100,000 and $350,000,...
-
On January 1, 2014, Vidalia Company issued 30,000 shares of $2 par value common stock for $150,000. On March 1, 2014, the company purchased 6,000 shares of its common stock for $8 per share for the...
-
On July 1, 2022, Burrough Company acquired 88,000 of the outstanding shares of Carter Company for $13 per share. This acquisition gave Burrough a 25 percent ownership of Carter and allowed Burrough...
-
The emf of the cell Ag|AgI(s)|AgI(aq)|Ag is +0.9509 V at 25C. Calculate (a) The solubility product of AgI and (b) Its solubility.
-
If the mitochondrial electric potential between matrix and the intermembrane space were 70 mV, as is common for other membranes, how much ATP could be synthesized from the transport of 4 mol H + ,...
-
A plane inclined at an angle of 45 passes through a diameter of the base of a cylinder of radius r. Find the volume of the region within the cylinder and below the plane (Figure 23).
-
The amounts of caffeine in a sample of five-ounce servings of brewed coffee are shown in the histogram. Number of 5-ounce servings S 25- 20 15 10 25 12 10 1 2 70.5 92.5 114.5 136.5 158.5 Caffeine (in...
-
Tom, David, Dale, and Murdock are four business students who want to rent a four- bedroom apartment together for the fall semester. They have identified the three factors important to them in...
-
Listed below, out of order, are the steps in an accounting cycle. 1. Prepare the unadjusted trial balance. 2. Post journal entries to general ledger accounts. 3. Analyze transactions from source...
-
Consider Quick Start QFD Matrix 2 above. Which two technical specifications are strongly correlated with each other? Quick Start QFD Matrix 2 Strong positive correlation Some positive correlation ==...
-
A cylindrical solenoid of length \(\ell\) and radius \(R\) has \(n\) windings per unit length and carries a current \(I\). (a) Use the inductance expression \(L=\left(\mu_{0} N^{2} A ight) / \ell\)...
-
What do you think of the potential for gaming products aimed at female consumers?
-
Explain the term "Equivalent Units". Why are they calculated in process costing? [4 Marks] [minimum 350 words]
-
In a double-glazed window, the panes of glass are separated by 1.0 cm. What is the rate of transfer of heat by conduction from the warm room (28 C) to the cold exterior (15 C) through a window of...
-
A lump of sucrose of mass 10.0 g is suspended in the middle of a spherical flask of water of radius 10 cm at 25 C. What is the concentration of sucrose at the wall of the flask after (a) 1.0h, (b)...
-
Confirm that is a solution of the diffusion equation with convection (eqn 19C.10) with all the solute concentrated at x=x 0 at t=0 and plot the concentration profile at a series of times to show how...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


