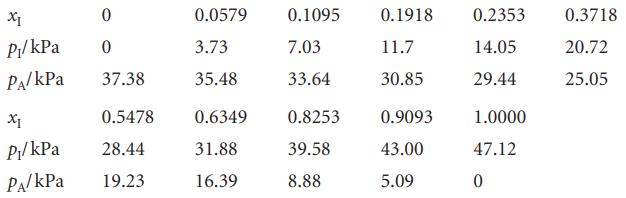
The table below lists the vapour pressures of mixtures of iodoethane (I) and ethyl acetate (A) at
Question:
The table below lists the vapour pressures of mixtures of iodoethane (I) and ethyl acetate (A) at 50°C. Find the activity coefficients of both components on
(a) The Raoult’s law basis,
(b) The Henry’s law basis with iodoethane as solute.
Transcribed Image Text:
XI 0 P₁/kPa 0 PA/kPa 37.38 X1 0.5478 P₁/kPa 28.44 PA/KPa 19.23 0.0579 3.73 35.48 0.6349 31.88 16.39 0.1095 7.03 33.64 0.8253 39.58 8.88 0.1918 11.7 30.85 0.9093 43.00 5.09 0.2353 14.05 29.44 1.0000 47.12 0 0.3718 20.72 25.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The table below lists gross domestic product (GDP), consumption (C), gross private domestic investment (I), government spending (G), and net exports (X M). Compute each as a percent of GDP for the...
-
The table below lists the cost per serving, in dollars, for items on four menus that are served at an elder-care facility. On a particular day, a dietician orders 32 meals from menu 1, 19 from menu...
-
The table below lists the cost per serving, in dollars, for items on three lunch menus served at a senior citizens' center. On a particular day, 26 Menu 1 meals, 18 Menu 2 meals, and 23 Menu 3 meals...
-
Willingham Company Ltd. has the following comparative statements of financial position data. WILLINGHAM COMPANY LTD. Statements of Financial Position December 31 Additional information for 2017: 1....
-
Compute the present value of the preceding cash flows.
-
List and explain five deceptive marketing practices, and explain how they are treated differently from offences against competition.
-
Ted is traveling on his railroad car (Fig. P27.5) with speed 0.85c relative to Alice. Ted travels for 30 s as measured on his watch. (a) Who measures the proper time, Ted or Alice? (b) How much time...
-
The T accounts below summarize the ledger of Bennet Landscaping Company at the end of the first month of operations.Instructions(a) Prepare the complete general journal (including explanations) from...
-
3. If you are offered to receive RM30,000 10 years from now in return for an investment of RM5,000 currently, what annual rate of return would you earn if you took the offer
-
Rusty Spears, CEO of Rusty's Renovations, a custom building and repair company, is preparing documentation for a line of credit request from his commercial banker. Among the required documents is a...
-
Consider a container of volume 5.0 dm 3 that is divided into two compartments of equal size. In the left compartment there is nitrogen at 1.0 atm and 25C; in the right compartment there is hydrogen...
-
The osmotic pressure of an aqueous solution at 300 K is 120 kPa. Calculate the freezing point of the solution.
-
The percentage of people with cell phones increased by 1.2 million people. Decide whether the statement makes sense (or is clearly true) or does not make sense (or is clearly false). Explain clearly.
-
Units processed during September for material and conversion. Ask an instructor lock lock lock A 3 A copy Determine the cost per equivalent unit for material and conversion cost combined. copy...
-
12% of all college students volunteer their time. Is the percentage of college students who are volunteers different for students receiving financial aid? Of the 338 randomly selected students who...
-
Mervon Company has two operating departments: mixing and bottling. Mixing has 3 3 0 employees and Bottling has 2 2 0 employees. Indirect factory costs include administrative costs of $ 1 8 2 , 0 0 0...
-
XP Ltd. is a manufacturing company with high stock requirements. Management are currently considering their stockholding policy. The following information is available for one stock item, material...
-
Process Costing: weighted average method Required: make a cost of production report in good form. Cost of Production Report-Weighted Average First Dept- Gem Company applies 100% of materials at the...
-
Interview the financial manager of a local business. What are the investment goals of this organization? What mix of securities does it use? What advantages and disadvantages do you see in its...
-
Represent each of the following combination of units in the correct SI form using an appropriate prefix: (a) m/ms, (b) k m, (c) k s /mg, and (d) k m N.
-
Suppose that a molecular orbital of a heteronuclear diatomic molecule is built from the orbital basis A, B, and C, where B and C are both on one atom (they can be envisaged as F2s and F2p in HF, for...
-
Sketch the molecular orbital energy level diagram for IF and deduce its ground-state electron configuration. Is IF likely to have a shorter bond length than IF or IF + ?
-
Why do both ionization energy and electron affinity play a role in estimating the energy of an atomic orbital to use in a molecular structure calculation?
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


