The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72
Question:
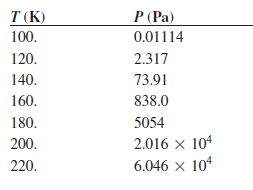
Transcribed Image Text:
T (K) P (Pa) 100. 0.01114 2.317 120. 73.91 140. 838.0 160. 180. 5054 200. 2.016 x 104 6.046 x 104 220.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
At the normal boiling point P 101325 Pa At the standard boiling point P 10 ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 298.15 K, G f (HCOOH, g) = -351.0 kJ mol -1 and G f (HCOOH, l) 361.4 kJ mol -1 . Calculate the vapor pressure of water at this temperature.
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
The vapor pressure of ethanol(l) is given by a. Calculate the standard boiling temperature. b. Calculate ÎH vaporization at 298 K and at the standard boiling temperature. 3.6745 x 10 23.58 In...
-
Determine the range of the 2x function y = 3 sec 3
-
Write a short Network Management Paper: In which, you will research and report on network management tools associated with: (1) Policy compliance, (2) Bandwidth management, (3) Asset management....
-
Find the mass and first moments about the coordinate axes of a thin square plate bounded by the lines x = 1, y = 1 in the xy-plane if the density is (x, y) = x 2 + y 2 + 1/3.
-
On January 1, 2010, Russell reacquires 8,000 of the outstanding shares of its own common stock for $24 per share. None of these shares belonged to Chapman. How does this transaction affect the parent...
-
Do you think global businesses would be willing to subscribe to a global code of conduct? Explain your answer.
-
What is the ending value (future value) of a one-year project that requires an initial investment of $100 and generates an interest rate of 5%
-
A production manager wants to assess the reactions of the blue-collar workers in his department (including foremen) to the introduction of computer-integrated manufacturing (CIM) systems. He is...
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
Benzene(l) has a vapor pressure of 0.1269 bar at 298.15 K and an enthalpy of vaporization of 30.72 kJmol 1 . The C P,m of the vapor and liquid phases at that temperature are 82.4 and 136.0 J K 1 mol...
-
Dale purchased Blue Corporation stock four years ago for $1,000 as an investment. He intended to hold the stock until funds were needed to help pay for his daughters college education. Today the...
-
Question (4) seen, 20 vehicles/km moving at 100 km/h and 30 vehicles/km traveling at 120 km/h. Two successive videos showing stationary traffic on the road were examined. Two groups of platoons were...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
How much water must be evaporated from 240 gallons of a 3% salt solution to produce a 5% salt solution?
-
Calculate the change in entropy when 100 kJ of energy is transferred reversibly and isothermally as heat to a large block of copper at (i) 0 C, (ii) 50 C.
-
Find the Fourier transform of the function f(x) = e |t| . Since this is an even function, you can use the one-sided cosine transform.
-
Construct a graph with the function f from the previous example and c 1 1 on the same graph. Let a = 1 for your graph. Comment on how well the partial sum with one term approximates the function.
-
Find the one-sided Fourier sine transform of the function ae bx .
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


