The vapor pressure of various substances can be determined using effusion. In this process, the material of
Question:
a. The best scale in your lab has an accuracy of ±0.01 g. What is the minimum amount of time you must wait until the mass change of the cell can be determined by your balance?
b. How much UF6 will remain in the Knudsen cell after 5.00 min of effusion?
Calculation of the collisional flux proceeds as follows:
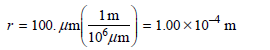
A = πr2 = π(1.00 × 10-4 m)2 = 3.14 × 10-8 m2
M = 0.352 kg mol -1 / NA = 5.85 × 10-25 kg
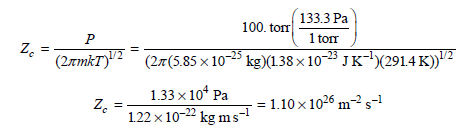
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





