The vapor pressures of 1-bromobutane and 1-chlorobutane can be expressed in the form And Assuming ideal solution
Question:
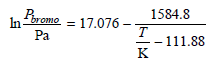
And
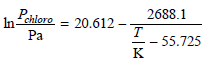
Assuming ideal solution behavior, calculate x bromo and y bromo at 305 K and a total pressure of 9750. Pa. At 305 K, Po bromo = 7113 Pa and Po chloro = 18552 Pa.
Transcribed Image Text:
1584.8 Poromo = 17.076 - In т 111.88 K Pa 2688.1 Pehlore Pa 20.612 – In- т - 55.725 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Ptotal Xbromo Promo 1 Xbromo Pehla c...View the full answer

Answered By

Shivani Gupta
I have done mtech from very reputed college .i am very passionate about teaching.i have more than 2 years of experience in teaching physics and prepared them for competitive exams like IIT-JEE and neet exams. I make physics easy to students as they think physics is difficult for them.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
x[n] is a real-valued, nonnegative, finite-length sequence of length N; i.e., x[n] is real and nonnegative for 0 n N 1 and is zero otherwise. The N-point DFT of x[n] is X[k], and the Fourier...
-
The sawtooth waveform in figure can be expressed in the form of a Fourier series as(a) Determine the Fourier series coefficients ck.(b) Use an N-point subroutine to generates samples of this signal...
-
The Heisenberg uncertainty principle can be expressed in the form where E represents energy and t represents time. Show that the units for this form are the same as the units for the form used in...
-
Alpha Appliance Service had net income for the year of $ 35,000. In addition, the balance sheet reports the following balances: Calculate the return on assets (ROA) for Alpha Appliance Service for...
-
Berry company manufactures two products, (1) Regular (2) Deluxe. The budgeted units to be produced are as follows: 2015 Regular Deluxe January 10,000 15,000 February 6,000 10,000 March 9,000 14,000...
-
Choose a story of organizational change, either from your textbook or through independent research, that was driven from within the organization. What was the impetus for change? What was the vision...
-
What are the main countries and sectors where Chinese FDIs have been concentrated in the last five years? What are the reasons behind these choices? LO.1
-
Youve completed your vacation in a foreign country. At the airport, you discover you have the equivalent of $20 local currency left over. The exchange control officer tells you that you cant convert...
-
Please help with Income statement, Balance sheet, and General Journal Required information [The following information applies to the questions displayed below.] The general ledger of Zips Storage at...
-
It is often said that consumers receive free content online. Is this the case? Why or why not?
-
A volume of 5.50 L of air is bubbled through liquid toluene at 298 K, thus reducing the mass of toluene in the beaker by 2.38 g. Assuming that the air emerging from the beaker is saturated with...
-
Construct a stem-and-leaf plot of the cholesterol changes? Table 2.15: Serum-cholesterol levels (mg/dL) before and after adopting a vegetarian diet *Before after. Subject Before After Difference* 195...
-
The density of water at 4oC is 1.00 X 103 kg/m3. What is waters density at 94oC?
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
The data in the table satisfy the equation y = k/x n , where n is a positive integer. Determine k and n. H y 2 1.5 3 1 4 0.75 5 0.6
-
Consider the activities undertaken by a medical clinic in your area. Required 1. Do you consider a job order cost accounting system appropriate for the clinic? 2. Identify as many factors as possible...
-
Calculate the standard enthalpies of formation of (a) KClO 3 (s) from the enthalpy of formation of KCl, (b) NaHCO 3 (s) from the enthalpies of formation of CO 2 and NaOH together with the following...
-
Calculate the standard enthalpy of solution of AgCl(s) in water from the enthalpies of formation of the solid and the aqueous ions.
-
When 120 mg of naphthalene, C 10 H 8 (s), was burned in a bomb calorimeter the temperature rose by 3.05 K. Calculate the calorimeter constant. By how much will the temperature rise when 10 mg of...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


