Use the Davies equation to calculate for a 1.00 molar solution of KOH. Compare your
Question:
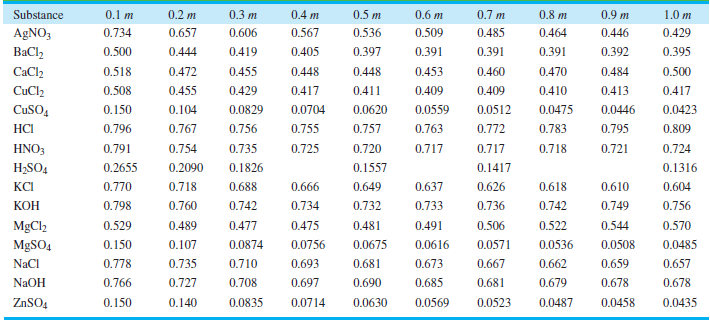
Use the Davies equation to calculate γ±for a 1.00 molar solution of KOH. Compare your answer with the values in Table 10.3.
Transcribed Image Text:
0.2 m 0.7 m 0.4 m 0,8 m 0.9 m Substance 0.1 m 0.3 m 0.5 m 0.6 m 1.0 m 0.657 0.485 AGNO3 0.734 0.606 0.567 0.536 0.509 0.464 0.446 0.429 0.405 0.391 BaCl, 0.500 0.444 0.419 0.397 0.391 0.391 0.392 0.395 CaCl2 0.518 0.472 0.455 0.448 0.448 0.453 0.460 0.470 0.484 0.500 0.508 0.411 0.409 0.413 CuCl2 0.455 0.429 0.417 0.409 0.410 0.417 0.0423 CuSO, 0.150 0.104 0.0829 0.0704 0.0620 0.0559 0.0512 0.0475 0.0446 0.755 0.763 0.783 0.795 НC 0.796 0.767 0.756 0.757 0.772 0.809 0.791 0.725 HNO3 0.754 0.735 0.720 0.717 0.717 0.718 0.721 0.724 H2SO4 0.2655 0.2090 0.1826 0.1557 0.1417 0.1316 КСі 0.770 0.718 0.688 0.666 0.649 0.637 0.626 0.618 0.610 0.604 0.798 КОН 0.760 0.742 0.734 0.732 0.733 0.736 0.742 0.749 0.756 0.475 MgCl2 0.529 0.489 0.477 0.481 0.491 0.506 0.522 0.544 0.570 0.0756 0.0616 MgSO4 0.150 0.107 0.0874 0.0675 0.0571 0.0536 0.0508 0.0485 0.693 0.673 NaCl 0.778 0.735 0.710 0.681 0.667 0.662 0.659 0.657 NAOH 0.766 0.697 0.727 0.708 0.690 0.685 0.681 0.679 0.678 0.678 0.140 0.0630 0.0569 0.0435 ZNSO4 0.150 0.0835 0.0714 0.0523 0.0487 0.0458
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
This answer is reasonably c...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of a buffer solution that is 0.200 molal in CH 3 COOH and 0.15 molal in CH 3 COONa using the Davies equation to calculate . What pH value would you have calculated if you had...
-
From pK1 and pK2 for glycine at = 0 in Table 9-1, compute pK1 and pK2 that apply at = 0.1 M. Use the Davies equation for activity coefficients. Compare your answer with experimental values in cells...
-
The solution containing no added KNO3 for Figure 7-1 contains 5.0 mM Fe(NO3)3, 5.0 M NaSCN, and 15 mM HNO3. We will use Davies activity coefficients to find the concentrations of all species in the...
-
Split the number 750 into two numbers x and y so that the sum of 8% of the first number and 24% of the second number is 11.2% of the sum of x and y.
-
Ingram Co. manufactures office furniture. During the most productive month of the year, 3,552 desks were manufactured at a total cost of $83,771. In its slowest month, the company made 1,243 desks at...
-
https://www.youtube.com/watch?v=PMiTVUDiNQ4 After watching this YouTube video, discuss what you just experienced and how we can apply what we have learned from this video to look for the constraints...
-
Outline the environment for international business. What dimensions need to be considered by organizations that operate internationally? LO.1
-
Software Distributors reports net income of $65,000. Included in that number is depreciation expense of $15,000 and a loss on the sale of land of $6,000. A comparison of this years and last years...
-
A 1000 bond is paying coupons at nominal interest rate of 7% payable semiannually. The bond is redeemed at par and matures on November 10, 2029. The nominal yield rate convertible semiannually is...
-
n Client Profile Ms. Fox is a 20-year-old black American who presents to the emergency department with complaints of chest pain and some shortness of breath. Ms. Fox indicates that she has had a...
-
What are the major differences between the positivist and interpretivist paradigms in consumer research?
-
Each country or region will have a core of products and services that are consumed. Collectively, the consumption of these infers some kind of group bond. Identify examples of these specific products...
-
In the reaction 2 A + B C + 3 D, reactant A is found to disappear at the rate of 6.2 x 10 4 M s 1 . (a) What is the rate of reaction at this point? (b) What is the rate of disappearance of B? (c)...
-
PAYMENTS DURING 2022/23 DATE DESCRIPTION FULL YEAR Private Hospital Insurance Premiums FULL YEAR Childcare costs FULL YEAR FULL YEAR FULL YEAR 18/08/22 27/08/22 01/09/22 01/10/22 01/11/22 01/12/22...
-
been called Recently, s asked to hington, ent of the in areas: g costs, and pany's a simple 12. The Tru-Green Lawn Company provides yard care services for customers throughout the Denver area. The...
-
During the week of November 12, 2021, Ernestina Manufacturing produced abd shipped 7,500 units of its aluminum wheels: 1,500 units of Model A and 6,000 units of Model B. The following costs were...
-
Daicos Ltd is a public company that competes in the highly competitive market for manufactured household products. The company is dominated by Peter Daicos, the chairman and chief executive officer,...
-
Hypothesis testing A tire company claims that a new range on average lasts at least 28,000 km. Tests with 64 tires result in an average duration of 27,800 km. With a standard deviation of 1,000 km....
-
Identify any horizontal or vertical asymptotes in the graph. State the domain of f. 564 X. 68 [
-
Avatar Financials, Inc., located on Madison Avenue, New York City, is a company that provides financial advice to individuals and small- to mid-sized businesses. Its primary operations are in wealth...
-
Label the regions of the phase diagram in Fig. 6.38. State what substances (if compounds give their formulas) exist in each region. Label each substance in each region as solid, liquid, or gas. Fig....
-
Haemoglobin, the red blood protein responsible for oxygen transport, binds about 1.34 cm 3 of oxygen per gram. Normal blood has a haemoglobin concentration of 150 g dm 3 . Haemoglobin in the lungs is...
-
K. Sato, F.R. Eirich, and J.E. Mark (J. Polymer Sci., Polym. Phys. 14, 619 (1976)) have reported the data in the table below for the osmotic pressures of polychloroprene ( = 1.25 g cm 3 ) in toluene...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


