Use the phase diagram in Fig. 6.40 to state (a) The solubility of Ag in Sn at
Question:
Use the phase diagram in Fig. 6.40 to state
(a) The solubility of Ag in Sn at 800°C and
(b) The solubility of Ag3Sn in Ag at 460°C,
(c) The solubility of Ag3Sn in Ag at 300°C.
Fig. 6.40
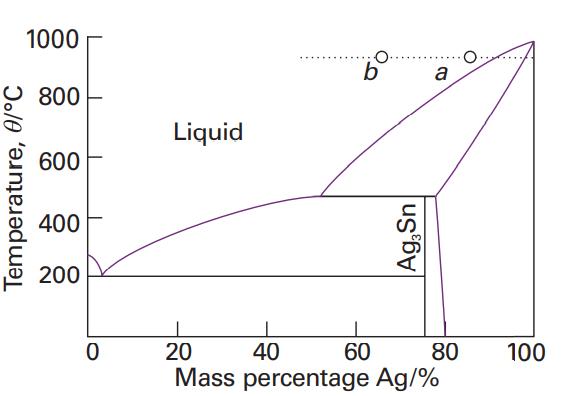
Transcribed Image Text:
Temperature, 0/°C 1000 800 600 400 200 0 Liquid O. b Ag,Sn 60 a 20 40 80 Mass percentage Ag/% 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
Solution a The solubility of Ag in Sn at 800C is given by x...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the phase diagram in Fig. 6.41 to state (a) The solubility of B in A at 500C and (b) The solubility of AB2 in A at 390C, (c) The solubility of ABz in Bat 300e.
-
The phase diagram for neon is Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will...
-
Use the phase diagram in Fig. 5.3 to state (i) the solubility of Ag in Sn at 800 C and (ii) the solubility of Ag 3 Sn in Ag at 460 C, (iii) the solubility of Ag 3 Sn in Ag at 300 C. Data in Fig. 5.3...
-
Passenger table (passengerid, address etc.) Flight table (flight id, departure, destination, depDate) Booking table (cID, fid, date, cost) a. Find Passengers who live in Chicago b. Total number of...
-
What is marking-to-market for a future? Why is this marking-to-market important for reducing counterparty risk?
-
Use reinforcement theory to explain why extrinsic rewards may not produce long-term effects on motivation. What should a leader do instead to motivate workers based on the relationship between...
-
Small binoculars or opera glasses (Fig. Q26.25) are useful in seeing the stage in detail. No prisms or mirrors are used, and yet the magnified image in these glasses is not inverted. Are the...
-
Mulberry Sporting Goods is authorized to issue 18,000 shares of common stock . During a two-month period, Mulberry completed these stock-issuance transactions: Apr 23 Issued 4,000 shares of $1.00 par...
-
Claudine Corporation will deposit $6,500 into a money market account at the end of each year for the next two years. How much will accumulate by the end of the second and final payment if the account...
-
Creating a Partnership Tax Return Overview You are a senior tax accountant in the firm, Ernest & Rainhouse. Joe, a new junior accountant, has just completed an interview with a new client Harry,...
-
Show that two phases are in mechanical equilibrium only if their pressures are equal.
-
Indicate on the phase diagram in Fig. 6.41 the feature that denotes incongruent melting. What is the composition of the eutectic mixture and at what temperature does it melt? Fig. 6.41 Temperature,...
-
You are in-house general counsel of Coulomb Co., a manufacturer of electrical equipment. In the ordinary course of business you are copied on emails regarding a purchase contract for several million...
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
As a leader, what are your highest values? o What's the contribution you want to make as a leader o What makes you distinct as a leader? o Drawing from StrengthsFinder 2.0 what are your strengths as...
-
What are local maximum and minimum values of a function?
-
Identify the source of funds within Micro Credit? How does this differ from traditional sources of financing? What internal and external governance mechanisms are in place in Micro Credit?
-
How is the method of combination differences used in rotation vibration spectroscopy to determine rotational constants?
-
The wavenumber of the J=32 rotational transition of 1 H 35 Cl considered as a rigid rotor is 63.56 cm 1 ; what is the HCl bond length?
-
Classify the following rotors: (i) O 3 , (ii) CH 3 CH 3 , (iii) XeO 4 , (iv) FeCp 2 (Cp denotes the cyclopentadienyl group, C 5 H 5 ).
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


