Use the phase diagram in Fig. 5.3 to state (i) the solubility of Ag in Sn at
Question:
Use the phase diagram in Fig. 5.3 to state (i) the solubility of Ag in Sn at 800 °C and (ii) the solubility of Ag3Sn in Ag at 460 °C, (iii) the solubility of Ag3Sn in Ag at 300 °C.
Data in Fig. 5.3
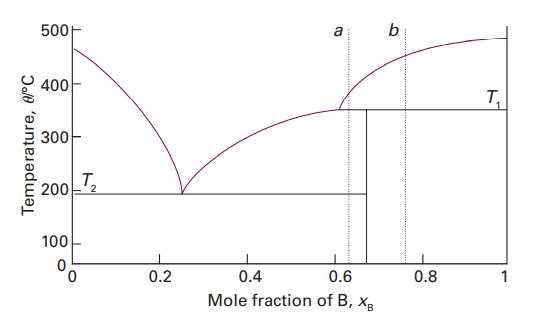
Transcribed Image Text:
Temperature, 0/°C 500 400 300 200 100 0 I _T₂ 0.2 a 0.4 0.6 Mole fraction of B, XB b 0.8 T₁ 1 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Answer i The solubility of Ag i...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Use the phase diagram in Fig. 6.41 to state (a) The solubility of B in A at 500C and (b) The solubility of AB2 in A at 390C, (c) The solubility of ABz in Bat 300e.
-
The phase diagram for neon is Temperature (K) Use the phase diagram to answer the following questions. (a) What is the approximate value of the normal melting point? (b) Over what pressure range will...
-
Use the phase diagram in Fig. 6.40 to state (a) The solubility of Ag in Sn at 800C and (b) The solubility of Ag 3 Sn in Ag at 460C, (c) The solubility of Ag 3 Sn in Ag at 300C. Fig. 6.40 Temperature,...
-
Rainbow manufactures wooden backyard playground equipment. Rainbow estimated $1,785,000 of manufacturing overhead and $2,100,000 of direct labour cost for the year. After the year was over, the...
-
Fredrick Wilson Company, an IFRS reporter, determined that one of its finite- life intangible assets is impaired. The assets net carrying value on the date of the impairment is $ 905,000. Fredrick...
-
What is the l-value of a variable? What is the r-value?
-
1. The tables are made of wood that costs $100 per table.
-
Ripkin Company issues 9%, five- year bonds dated January 1, 2013, with a $ 320,000 par value. The bonds pay interest on June 30 and December 31 and are issued at a price of $ 332,988. Their annual...
-
Saxon Products, Inc., is investigating the purchase of a robot for use on the companys assembly line. Selected data relating to the robot are provided below: Cost of the robot $ 1,750,000...
-
Identify the unusual values of x in each histogram in Exercises 35. Data from exercises 3-5: 3. 4. 5. P(x) P(x) P(x) 0.40 0.40 0.40 0.30 0.20 0.30 0.30 0.20 0.10 0.20 0.10 0.10 o i 2 3 4 5 0 1 2 3 4...
-
The temperaturecomposition diagram for the Ca/Si binary system is shown in Fig. 5.7. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b) If a 20 per cent by...
-
Referring to Fig. 5.8, deduce the molar solubility of (i) NH 4 Cl, (ii) (NH 4 ) 2 SO 4 in water at 25 C. Data in Fig. 5.8, 0.8 NHCI 10 0.6 0.2 0.44 0.2 P=2 0/1 HO P=1 fg P=3 0.4 0.8 P=2 0.6 0.6 0.4...
-
How do the eight general environmental factors affect the five industry forces?
-
Explain the way to generate value of business for business sustainable development with one example (5 points) Identify and explain the key points for positive results for business sustainable...
-
Systematization is the most common way of causing specific practices and ways of behaving to turn out to be solidly settled inside an association or society. It includes making clear principles,...
-
Imagine that you are working at Seneca Bank as a Financial Planner. You are working hard to establish your Financial Planning practice and you realize there are many aspects involved in successfully...
-
Consider all of the analytical tools that Marr has presented. Choose 3 and research ways that specific businesses (after 2022 and other than those that Marr has mentioned) have used each of the...
-
First, tell us about an organization that you thoroughly enjoyed working for and explain what the dynamics of that culture was like. Please go into detail. Second, tell us about an organization that...
-
Suppose that we start a major scale on concert A, which is defined to have a frequency of 440 Hz. If we call this frequency do, what is ideal-ratio frequency of a. mi? b. sol?
-
What is the expected payoff of an investment that yields $5,000 with a probability of 0.15 and $500 with a probability of 0.85? Select one: O a. $325 O b. $5,500 O c. $2,750 O d. $1,175
-
Discuss the following statement: If the temperature of the system increased, heat must have been added to it.
-
Oxygen reacts with solid glycylglycine C 4 H 8 N 2 O 3 to form urea CH 4 N 2 O, carbon dioxide, and water: 3O 2 (g) + C 4 H 8 N 2 O 3 (s) CH 4 N 2 O(s) + 3CO 2 (g) + 2H 2 O(l) At T = 298 K and 1.00...
-
Discuss the following statement: Heating an object causes its temperature to increase.
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


