Referring to Fig. 5.8, deduce the molar solubility of (i) NH 4 Cl, (ii) (NH 4 )
Question:
Referring to Fig. 5.8, deduce the molar solubility of (i) NH4Cl, (ii) (NH4)2SO4 in water at 25 °C.
Data in Fig. 5.8,
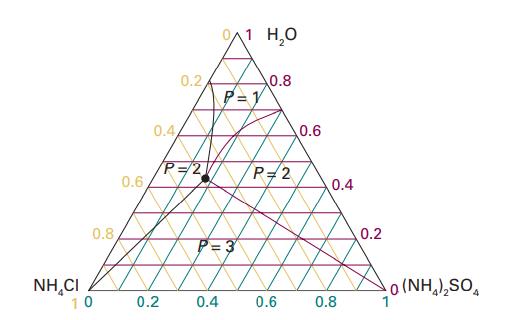
Transcribed Image Text:
0.8 NHẠCI 10 0.6 0.2 0.44 0.2 P=2 0/1 H₂O P=1 fg P=3 0.4 0.8 P=2 0.6 0.6 0.4 0.8 0.2 0(NH,),SO.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Answer i The molar solubilit...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The molar solubility of MnCO3 is 4.2 3 1026 M. What is Ksp for this compound?
-
The diffusion coefficient of glucose in water at 25 C is 6.73 10 10 m 2 s 1 . Estimate the time required for a glucose molecule to undergo a root-mean-square displacement of 5.0mm.
-
Liquid water at 25 C and 1 bar fills a rigid vessel. If heat is added to the water until its temperature reaches 50 C, what pressure is developed? The average value of ( between 25 and 50oC is 36.2 (...
-
Mickey Limited is a manufacturing business that uses a standard costing system. The companys flexed budget for April 20X9 is: Sales 173,340 Costs Direct materials (22,500) Direct labour (37,684)...
-
Perlu Products, an IFRS reporter, reported an impairment loss of $ 65,000 for one of its plant assets on December 31, 2015. At December 31, 2016, the assets recoverable amount increased by $ 90,000....
-
Describe a situation when a history-sensitive variable in a subprogram is useful.
-
2. The tables are assembled by workers, at a wage cost of $40 per table.
-
What is the difference between preparing the statement of cash flows using the direct method and using the indirect method?
-
HMK Enterprises is considering two proposals to overhaul its network infrastructure. They have received two bids. The first bid, from Huawei, will require a $22 million upfront investment and will...
-
Julio produces two types of calculator, standard and deluxe. The company is currently using a traditional costing system with machine hours as the cost driver but is considering a move to...
-
Use the phase diagram in Fig. 5.3 to state (i) the solubility of Ag in Sn at 800 C and (ii) the solubility of Ag 3 Sn in Ag at 460 C, (iii) the solubility of Ag 3 Sn in Ag at 300 C. Data in Fig. 5.3...
-
Air is a mixture with mass percentage composition 75.5 (N 2 ), 23.2 (O 2 ), 1.3 (Ar). Calculate the entropy of mixing when it is prepared from the pure (and perfect) gases.
-
An intervention program designed by the Stockholm Transit District was implemented to improve the work conditions of the citys bus drivers. Improvements were evaluated by G. Evans et al., who...
-
Write down at leastfive items (durable goods, not food) that you purchase and their sourcing (where each is from). For example, a shirt may be assembled in China, designed in the US, and made from...
-
I agree that overtime can be tricky in different countries. As we've seen with piecework, it is hard to implement it in countries where people are not motivated to work past their regular hours, even...
-
time (in seconds). Find a formula for 1, if V = 5t(t Suppose that an object's acceleration function is given by a = 4t+ 6. The object's initial velocity is 4, and the initi position is 9. Find the...
-
Determine the specific major and foundational managerial discoveries and findings from each era as most pivotal for management evolution (Early Management Era, Social Management Era, Scientific...
-
Identify the three major pricing strategies and discuss the important key factors that impact setting prices. Explain what time of pricing strategy your assigned brand uses and why you believe this...
-
An organ pipe closed at one end and open at the other has a length of 0.5 m. a. What is the longest possible wavelength for the interfering sound waves that can form a standing wave in this pipe? b....
-
You are interested in investing and are considering a portfolio comprised of the following two stocks. Their estimated returns under varying market conditions are provided: (note: it is difficult to...
-
The Joule coefficient is defined by (T /V) U = (1/C V )[P T(P/T) V ]. Calculate the Joule coefficient for an ideal gas and for a van der Waals gas.
-
What is the physical origin of the pressure difference across a curved liquidgas interface?
-
Using the result of Equation (3.8), (P/T) V = / , express as a function of and V m for an ideal gas, and as a function of b, , and V m for a van der Waals gas.
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


