Use the vapor pressures for hexane given in the following table to estimate the temperature and pressure
Question:
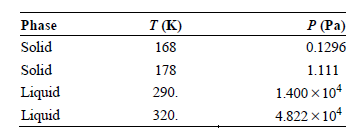
Transcribed Image Text:
Phase T (K) 168 P (Pa) 0.1296 Solid Solid Liquid Liquid 178 1.111 1.400 x 104 4.822 x 104 290. 320.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
To calculate the triple point temperature take subli...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures for tetrachloromethane given in the following table to estimate the temperature and pressure of the triple point and also the enthalpies of fusion, vaporization, and...
-
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical method or a least squares fitting routine. T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230....
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Midland Corporation has a net income of $19 million and 4 million shares outstanding. Its common stock is currently selling for $48 per share. Midland plans to sell common stock to set up a major new...
-
In 2014, Toyota faced significant sanctions in the case of Toyota's Recall Problems. Summarize those sanctions and comment on whether you believe they were minimal, adequate, or excessive.
-
The combined cycle plant shown in Fig. 9.36 uses a gas-turbine engine operating a Brayton cycle with air as the working fluid. The air enters the compressor at 102 kPa and 15 C and exits at 612 kPa....
-
Explain why taxes, fines and other transfers are non-exchange transactions.
-
An experiment was conducted to investigate leaking current in a SOS MOSFETS device. The purpose of the experiment was to investigate how leakage current varies as the channel length changes. Four...
-
Sonu, Sona and Seema together started a tailoring centre. Their investment was in the ratio 5:7:8. They divided one years profit in the same ratio. Seema got 5000 rupees more than Sona. How much did...
-
Schrand Aerobics, Inc., rents studio space (including a sound system) and specializes in offering aerobics classes. On January 1, 2019, its beginning account balances are as follows: Cash,...
-
Which two business concepts are most highly correlated? a. Risk and reward b. Risk and revenue c. Reward and investment d. Investment and revenue
-
Show the paths n o p q and a b c d e f of the PVT phase diagram of Figure 8.15 in the PT phase diagram of Figure 8.4. Figure 8.4 Figure 8.15 Critical - point Liquid Solid Triple point Gas Tm...
-
Complete the following table, and use the results to discuss when the rule of 70 gives a better approximation for the doubling time, and when the rule of 72 gives a better approximation. r (In...
-
1. Define a person-centered model of care in LTC facilities. 2. Describe two leadership behaviors and two leadership qualities most conducive to moving long-term care organizations toward more...
-
question 5 all parts 8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin...
-
3- Define and describe, in detail, the various communication styles as they relate to negotiation and conflict resolution. Compare the advantages and disadvantages of the styles. Provide a detailed...
-
SJ Corp ahs the following data for 2020: RM, beginning of 5,000; Purchases of raw materials is 50,000; return of defective raw materials to suppliers of 4,000; return of direct materials from the...
-
A company is issuing $340,000 worth of 4-year bonds on October 8, 2023, bearing an interest rate of 2%, payable annually. Assume that the current market rate of interest is 3%. a) Will the bonds be...
-
Solve the rational inequality (x + 1) x-2 VI 0
-
Assume Eq. 6-14 gives the drag force on a pilot plus ejection seat just after they are ejected from a plane traveling horizontally at 1300 km/h. Assume also that the mass of the seat is equal to the...
-
The Hückel secular equation for the hydrogen molecule is Determine the two orbital energies in terms of α and β. = 0.
-
Find the eigenvalues and eigenvectors of the matrix 10 1 1 1 0
-
Find the eigenvalues and eigenvectors of the matrix 111 1 1
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


