Use the vapor pressures of n-butane given in the following table to calculate the enthalpy of vaporization
Question:
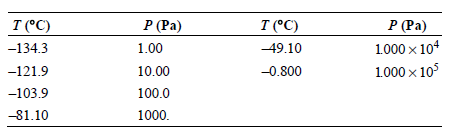
Transcribed Image Text:
P (Pa) 1000 x 104 1000 x 105 T (°C) -134.3 P (Pa) T (°C) 49.10 1.00 -0.800 -121.9 10.00 -103.9 100.0 -81.10 1000.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
A least squares fit of ...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
Amandeep, a graduate of Cambrian Colleges Business Program, found a job as an online marketing specialist for Tangerine Bank. Within a year, he had saved {A} and decided to buy a new car but was not...
-
Read the Case study (Proctor & Gamble) and answer the following: 1. In your own words, summarize what went RIGHT or WRONG in this case? 2. Describe the Project Management process and practices used...
-
Find the lines x- and y-intercepts and use this information to graph the line. 3x + 4y = 12
-
List the economic conditions (inflation, current interest rates) and personal factors related to the costs and benefits of financial services that you should monitor as your personal life situation...
-
The Thorndikes have submitted a bid to be the sole supplier of swimming goggles for the U.S. Olympic team. OptiView, Inc. has been supplying the goggles for many years, and the Olympic committee has...
-
Emanuel, the owner of Best Enterprise set up a business selling books in his hometown. The Prepare the Statement of Comprehensive Income for the year ended and Statement of Financial Position as at...
-
What is the maximum number of super keys for the relation R1(P, Q, R, S) with R as the key? () 6 (b) 8 () 16 (d) 32
-
The variation of the vapor pressure of the liquid and solid forms of a pure substance near the triple point are given by ln P solid /Pa = -8750K/T + 34.143 and In P liquid /Pa = -4053K/T + 21.10....
-
At 298.15 K, G f (HCOOH, g) = -351.0 kJ mol -1 and G f (HCOOH, l) 361.4 kJ mol -1 . Calculate the vapor pressure of water at this temperature.
-
One way of thinking about organizing to implement cost leadership strategies is that firms that pursue this strategy should be highly centralized, have high levels of direct supervision, and keep...
-
Context This task requires analysing a network scenario, design the network architecture and recommend IT solutions including ethical, security and sustainability considerations.The purpose of this...
-
What was the Prime Cost Percent for Mandy's BBQ Pit for August? Select one: a. 46.5% b. 73.9% c. 63.4% d. 85%
-
Finding Critical Values and Confidence Intervals. In Exercises 5-8, use the given information to find the number of degrees of freedom, the critical values x? and x*, and the confidence interval...
-
An investor sold 100 shares of ABC stock short at $25 and buys one ABC Jan 30 call @ $5. What is this investor's maximum gain, maximum loss, and breakeven points from this strategy?
-
Jake, Sachs and Brianne own a tour company called Adventure Sports. The partners share profits and losses in a 1:3:4 ratio. After Lengthy Dissagreements among the partners and several unprofitable...
-
In Exercises 7172, use the graph of the polynomial function to solve each inequality. f(x) = 2x + 11x 7x 6 [-7, 3, 1] by [-10, 70, 10]
-
In Exercises 1558, find each product. (9 - 5x) 2
-
An object moves through a fluid in the x direction. The only force acting on the object is a frictional force that is proportional to the negative of the velocity: Write the equation of motion of the...
-
Repeat the calculation of the previous example with a = 0.500 s 1 . Show that a narrower line width occurs.
-
Find the one-sided Fourier sine transform of the function f(x) = x.
-
Problem Set Time Value of Money 1. In 10 years, what is the value of $100 invested today at an interest rate of 8% per year, compounded annually? 2. In 10 years, what is the value of $100 invested...
-
The Blending Department of Luongo Company has the following cost and production data for the month of April. Costs: Work in process, April 1 Direct materials: 100% complete $120,000 Conversion costs:...
-
Q3 plz answer correctly and check work Builtrite's upper management has been comparing their books to industry standards and came up with the following question: Why is our operating profit margin...

Study smarter with the SolutionInn App


