Using Table 22.3, which lists the possible terms that arise from a given configuration, and Hunds rules,
Question:
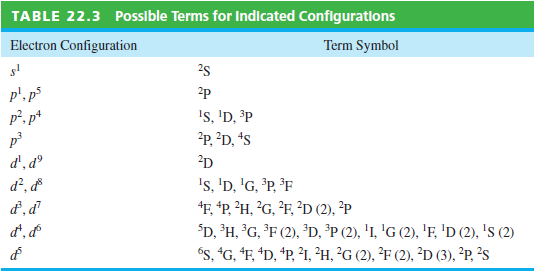
Transcribed Image Text:
Possible Terms for Indicated Configurations TABLE 22.3 Term Symbol Electron Configuration 25 p'. p p², p* 2p Is, 'D, ³P ?p, ²D, *s d', dº 2D d², d 's, 'D, 'G, ³P, ³F F, *P, ?H, ?G, ?F, ?D (2), ?P "D, ®H, °G, °F (2), ³D, ³P (2), 'I, 'G (2), 'F, 'D (2), 's (2) és, "G, "F, *D, *P, ?1, ²H, ?G (2), ²F (2), ²D (3), ?P, ²s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
We use Hunds rule that the term with the highest multiplicity is the lowest in energy to ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The configuration for the ground state of iridium is [Xe]4f145d76s2. What are the group and period for this element? Is it a main-group, a d-transition, or an f-transition element?
-
Write the orbital diagram for the ground state of cobalt. The electron configuration is [Ar]3d74s2.
-
Write the orbital diagram for the ground state of terbium. The electron configuration is [Xe]4f96s2.
-
What are some Marketing Strategies for Delivering Objectives Polestar (Car company) has used or using?
-
Sketch the graph of the function. (Include two full periods.) 1. y = sin 6x 2. y = -cos 3x 3. f (x) = 5 sin(2x / 5) 4. f (x) = 8 cos ( -x / 4) 5. y = 5 + sin x
-
Use Cramers rule to solve the systems in Problems 2132. 6x + 7y = 3 8x + 9y = 4
-
Refer to Example 11.2 and the simple linear regression of the drug reaction data in Table 11.1. a. Compute an estimate of s. b. Give a practical interpretation of the estimate. LO9
-
Member AB is supported at B by a cable and at A by a smooth fixed square rod which fits loosely through the square hole of the collar. If F = {20i ? 40j ? 75k}, determine the x, y, z components of...
-
On July 1, 2020, Cheyenne Corporation issued $3,500,000 of 9% bonds payable in 20 years. The bonds include detachable warrants giving the bondholder the right to purchase for $30 one share of $1 par...
-
Please solve this problem using C language Hacker Industries has a number of employees. The company assigns each employee a numeric evaluation score and stores these scores in a list. A manager is...
-
The Doppler broadening in a gas can be expressed as where M is the molar mass. For the sodium transition, ν 0 5.0933 à 10 14 s -1 . Calculate Îν and...
-
What J values are possible for a 6 H term? Calculate the number of states associated with each level and show that the total number of states is the same as that calculated from the term symbol.
-
What is the effect if the requirements of the Statute of Frauds are not met? Will anything else other than actual writing satisfy the requirements of the Statute of Frauds?
-
Problem 2.01 An ant is crawling along a straight wire, which we shall call the x axis, from A to B to C to D (which overlaps A), as shown in the figure below. O is the origin. Suppose you take...
-
In a separate C++ program, do the following: a) Create an unordered linked list by declaring a linked list of the unordered LinkedList type. You may assume that this list is to be comprised of...
-
TranscribedText: El. You are sitting at a table that has a solid round top {5.1"} kg] and a single solid cvlindrical leg {4. kg) [see figure, note that the tilt angle is exaggerated to he...
-
The municipal mill rate in the neighbourhood is 22.375 mills. There is an educational mill rate of 11.35 mills. The following list is the municipalities planned local improvement costs for the next...
-
Maggie Company had the following functional income statement for the month of May, 2020: MAGGIE COMPANY Functional Income Statement For the Month Ending May 31, 2020 Sales (30,000 units) $300,000...
-
In Exercises 4144, write a function in slope-intercept form whose graph satisfies the given conditions. Passing through (-1, -5) and (2, 1)
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
Calculate the standard enthalpy of solution of AgCl(s) in water from the enthalpies of formation of the solid and the aqueous ions.
-
When 120 mg of naphthalene, C 10 H 8 (s), was burned in a bomb calorimeter the temperature rose by 3.05 K. Calculate the calorimeter constant. By how much will the temperature rise when 10 mg of...
-
A sample consisting of 1.00 mol of a van der Waals gas is compressed from 20.0 dm 3 to 10.0 dm 3 at 300 K. In the process, 20.2 kJ of work is done on the gas. Given that = {(2a/RT) b}/C p,m, with C...
-
Justice Corporation Comparative Balance Sheet December 31, 2025 and 2024 2025 2024 Assets Current Assets: $ Cash and Cash Equivalents 2,254 $ 1,876 Justice Corporation reported the following...
-
The Fields Company has two manufacturing departments forming and painting. The company uses the FIFO method of process costing at the beginning of the month the forming department has 33.000 units in...
-
A comparative balance sheet for Lomax Company containing data for the last two years is as follows: Lomax Company Comparative Balance Sheet This Year Last Year $ 96,000 $ 70,000 640,000 672,500...

Study smarter with the SolutionInn App


