Question: Using Table 22.3, which lists the possible terms that arise from a given configuration, and Hunds rules, write the term symbols for the ground state
Using Table 22.3, which lists the possible terms that arise from a given configuration, and Hund€™s rules, write the term symbols for the ground state of the atoms H through F in the form(2s+1)LJ.
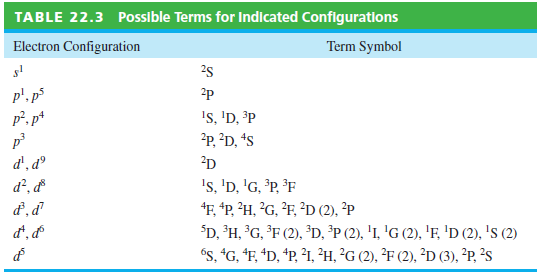
Possible Terms for Indicated Configurations TABLE 22.3 Electron Configuration Term Symbol 25 p', p5 p?. p* 2p Is, 'D, P ?p, D, *s 2D d', d 's, 'D, 'G, P, F F, *P, ?H, ?G, ?F, ?D (2), ?P $D, H, G, F (2), D, *P (2), 'I, 'G (2), 'F, 'D (2), 's (2) "s, "G, "F, *D, *P, I1, H, ?G (2), F (2), D (3), *P, ?s d?, d d, d
Step by Step Solution
3.33 Rating (153 Votes )
There are 3 Steps involved in it
We use Hunds rule that the term with the highest multipli... View full answer

Get step-by-step solutions from verified subject matter experts


