Using the formula for the energy levels for the Morse potential, show that the energy spacing between
Question:
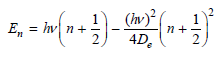
show that the energy spacing between adjacent levels is given by
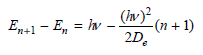
Transcribed Image Text:
(hv)² n + E, = hv n+ ADE En+1 - E, = iw 2D. (in² (n + 1) (Iv)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
Eus E tv x 2 x x x 1 hvn 3 hv ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the Bohr formula for the energy levels, calculate the energy required to raise the electron in a hydrogen atom from n = 1 to n = . Express the result for 1 mol H atoms. Because the n = level...
-
Calculate the ionization energy of the He+ ion in kJ/mol (this would be the second ionization energy of He). See Problem 8.107. The Bohr formula for the energy levels of an ion consisting of a...
-
Using the formula for the moment of inertia of a uniform sphere, find the moment of inertia of a thin spherical layer of mass m and radius R relative to the axis passing through its centre.
-
What are four advantages of integrating AI technologies into decision support systems?
-
Consider the function f (x) = 3 sin (0.6x 2). (a) Approximate the zero of the function in the interval [0, 6]. (b) A quadratic approximation agreeing with f at x = 5 is g (x) = -0.45x2 + 5.52x -...
-
In Problems 1728, determine which functions are polynomial functions. For those that are, state the degree. For those that are not, state why not. Write each polynomial in standard form. Then...
-
If the opening backlog is 500 units, forecast demand is 700 units, and production is 800 units, what will be the ending backlog? LO.1
-
The weekly time tickets indicate the following distribution of labor hours for three direct labor employees: The direct labor rate earned per hour by the three employees is as follows: Tom Couro...
-
D Question 15 Gant Motorcycles Inc. pays a $3.08 preferred dividend every year and will maintain this policy forever. What price should you pay for one share of preferred stock if you want an annual...
-
Use the data in MURDER.RAW for this exercise. The variable mrdrte is the murder rate, that is, the number of murders per 100,000 people. The variable exec is the total number of prisoners executed...
-
A measurement of the vibrational energy levels of 12 C 16 O gives the relationship where n is the vibrational quantum number. The fundamental vibrational frequency is ν 0 = 2170.21 cm...
-
Use your results from P19.36 to solve the following problem. For 1 H 35 Cl, D e = 7.41 10 -19 J and = 8.97 10 13 s -1 . As n increases, the energy difference between adjacent vibrational levels...
-
Figure 405 gives the impression that the potential on the y-axis changes more rapidly near 0 than near i. Can you verify this? y P 3 kV 12 1 i-1 -2 -3 P:-3 kV Fig. 405. Example 2: z-plane
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
In Exercises 2738, evaluate each function at the given values of the independent variable and simplify. a. b. c. d. h(x) = x4 x + 1
-
Periwinkle Company is a multinational organization. Its Parts Division is located in Lavender Land, while its Assembly Division is located in North Orchid. During the current year Periwinkle Companys...
-
Air in industrial plants is subject to contamination by many different chemicals, and companies must monitor ambient levels of hazardous species to be sure they are below limits specified by the...
-
An adult takes about 12 breaths per minute, inhaling roughly 500 mL of air with each breath. The molar compositions of the inspired and expired gases are as follows: The inspired gas is at 24C and 1...
-
A spray-drying operation similar to that described in Problem 5.23 is used by the pharmaceutical industry. Acetaminophen is an active pharmaceutical ingredient (API) that can be produced in powdered...
-
Problem 12.6A (Algo) Liquidation of a partnership LO P5 Kendra, Cogley, and Mel share income and loss in a 3.21 ratio (in ratio form: Kendra, 3/6: Cogley, 2/6; and Mel, 1/6), The partners have...
-
Melody Property Limited owns a right to use land together with a building from 2000 to 2046, and the carrying amount of the property was $5 million with a revaluation surplus of $2 million at the end...
-
Famas Llamas has a weighted average cost of capital of 9.1 percent. The companys cost of equity is 12.6 percent, and its cost of debt is 7.2 percent. The tax rate is 25 percent. What is the companys...

Study smarter with the SolutionInn App


