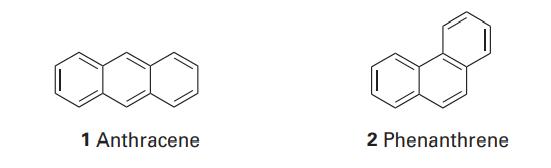
Write down the secular determinants for (i) anthracene (1), (ii) phenanthrene (2) within the Hckel approximation and
Question:
Write down the secular determinants for (i) anthracene (1), (ii) phenanthrene (2) within the Hückel approximation and using the C2p orbitals as the basis set.
Transcribed Image Text:
1 Anthracene 2 Phenanthrene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Thorough Explanation The secular determinants for anthracene 1 and phenanthrene 2 within t...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Write down the secular determinants for (i) linear H 4 , (ii) cyclic H 4 within the Hckel approximation.
-
Write down the secular determinants for (a) Linear H 3 , (b) Cyclic H 3 within the Hckel approximation.
-
Write down abbreviated expressions for the remaining six determinants of the N2 VB function of Section 13.12. Use the rule given in that section to find the coefficient of each determinant in the...
-
Consider the following population regression model: y = Bo + Bx + Bx2 + 3x3 + u Suppose you want to test whether 0.532 = 83. The hypotheses are: Ho : 0.582 = 33 H : 0.532 #33 The correct expression...
-
For a batch processing system using sequential files, describe the intermediate and permanent files that are created after the edit run has successfully been completed when processing the sales order...
-
What does the following program print? // Exercise 4.26: Mystery3.java 2 public class Mystery3 { 3 public static void main(String [] args) { 4 5 int row = 10; 6 while (row >= 1) { 7 int column - 1; 8...
-
Why do project teams create time-phased budgets? What are their principal strengths?
-
Alcoa Inc. is the worlds largest producer of aluminum products. One product that Alcoa manufactures is aluminum sheet products for the aerospace industry. The entire output of the Smelting Department...
-
What is the yield to maturity on an 18-year, zero coupon bond selling for 35% of par value? 1) 6.92% 2) 6.01% 3) 6.37% 4) 5.86% 5) 4.86%
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The computer center at Rock-bottom University has been experiencing computer downtime. Let us assume that the trials of an associated Markov process are defined as one-hour periods and that the...
-
What is the physical significance of the Coulomb and resonance integrals?
-
The owners of an oil reserve begin extracting oil at time t = 0. Based on estimates of the reserves, suppose the projected extraction rate is given by Q'(t) = 3t 2 (40 - t) 2 , where 0 t 40, Q is...
-
460 V rms 3 phase full wave controlled rectifier feeds an inductive load. The supply voltage has a frequency of 50 Hz. If thyristors are considered ideal; a) Draw the voltage on the load when a = 25....
-
The following data is provided for Garcon Company and Pepper Company for the year ended December 31. Garcon Company Pepper Company Finished goods inventory, beginning $14,000 $17,950 Work in process...
-
On September 22, 2024, a flood destroyed the entire merchandise inventory on hand in a warehouse owned by the Rocklin Sporting Goods Company. The following information is available from the records...
-
A wound DC motor is connected in both a shunt and a series configuration. Assume generic resistance and inductance parameters Ra, Rf, La, Lf, let the field magnetization constant be kf and the...
-
Supermart Food Stores (SFS) has experienced net operating losses in its frozen food products line in the last few periods. Management believes that the store can improve its profitability if SFS...
-
Evaporation and condensation are always happening. List, from first to last, the following events that lead to the formation of water droplets: (a) the relative humidity approaches 100%, (b) the...
-
A certain Christmas tree ornament is a silver sphere having a diameter of 8.50 cm. Determine an object location for which the size of the reflected image is three-fourths the size of the object. Use...
-
How do you expect S m for an ion in solution to change as the ionic radius increases at constant charge?
-
How do you expect S m for an ion in solution to change as the charge increases at constant ionic radius?
-
It takes considerable energy to dissociate NaCl in the gas phase. Why does this process occur spontaneously in an aqueous solution? Why does it not occur spontaneously in CCl 4 ?
-
Apple inc cash flow
-
Assume todays settlement price on a CME EUR futures contract is $1.3142 per euro. You have a short position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Determining ending consolidated balances in the second year following the acquisition-Equity method Assume that your company acquired a subsidiary on January 1, 2012. The purchase price was $650,000...

Study smarter with the SolutionInn App


