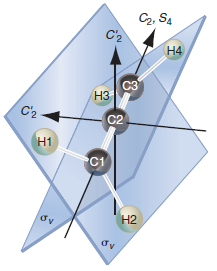
XeF 4 belongs to the D 4h point group with the following symmetry elements: E, C 4
Question:
Transcribed Image Text:
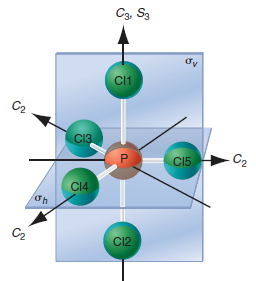
Сг, SA C2 Н4 сЗ НЗ C'2 C2 H1 C1 Н2 Сз Sg бy C1 C2 C13 св C15 - C2 C14 C12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The fourfold axis is perpendicular to the plane of th...View the full answer

Answered By

Mary Njunu
I posses Vast, diversified knowledge and excellent grammar as a result of working in ACADEMIC WRITING for more than 5 years. I deliver work in various disciplines with assurance of quality work. I purpose at meeting the clients’ expectations precisely. Let’s work together for the best and phenomenal grades.
4.90+
929+ Reviews
2557+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
CH 4 belongs to the T d point group with the following symmetry elements: E, 4C 3 , 4C 2 3 , 3C 2 , 3S 4 , 3S 3 4 , and 6Ï. Make a drawing similar to Figure 27.1 showing these elements. C2, S....
-
List the symmetry elements of the following molecules and name the point groups to which they belong: (a) Staggered CH3CH3, (b) Chair and boat cyclohexane, (c) B, H6, (d) [Co (en) 3J3+, where en is...
-
The CCl4 molecule belongs to the point group Td. List the symmetry elements of the group and locate them in the molecule.
-
1.Alice is your long-term friend from high school. Alice was typically quieter in the group, but she was well liked amongst your peers. In high school she began to come out of her shell a little...
-
Consider the graph of y = ekt. Describe the characteristics of the graph when k is positive and when k is negative.
-
Suppose the writer had chosen to write a how-to article for people wanting to change their hair color. Would this essay fulfill the writers goal? A. NO CHANGE B. Along with C. Or D. As well as
-
2-3. What is the meaning of an organizations mission?
-
Facts: Your client is the plaintiff in a workers' compensation case. She was injured in 1993 in state A. In 1995, her employer destroyed all the business records relating to the client. The...
-
Use the FIFO/LIFO Inventory grids by clicking on the GRIDS tab below. June 1 each 4 each 6 8 14 Balance Purchased Sold Purchased Sold Purchased Sold Purchased Sold 20 units 10 units 15 units 20 units...
-
The following accounts are taken from the records of Jasper Inc. at January 31, 2015, its first month of operations. Required: 1. Calculate the amount of total assets. 2. Calculate the amount of...
-
NH 3 belongs to the C 3v group. The reducible representation for the vibrational modes is reducible = 2A 1 + 2 E . a. How many vibrational modes does NH 3 have? b. How many of these modes are...
-
Methane belongs to the T d group. The reducible representation for the vibrational modes is reducible = A 1 + E + 2T 2 . a. Show that the A 1 and T 2 representations are orthogonal to each other and...
-
What legal responsibility does an organization have to protect against semantic security problems?
-
On July 1, 2021, P Company borrowed P160,000 to purchase 80 percent of the outstanding common stock of S Company. This loan, carrying a 10 percent annual rate, is payable in 8 annual installments...
-
Case Analysis Strategic leaders, being at the highest level of an organization, are responsible for charting its path to success. They visualize an ideal picture of their enterprise in a futuristic...
-
3 Refrigerant-134a enters a adiabatic compressor at 100 kPa and -24C with a flow rate of 1.300 m/min and leaves at 800 kPa and 60C. Determine the mass flow rate of R-134a and the power input to the...
-
The following trial balance of Bramble Traveler Corporation does not balance. Bramble Traveler Corporation Trial Balance April 30, 2025 Debit Credit Cash $6,221 Accounts Receivable 5,350 Supplies...
-
From this analysis, we can see than the actual number of unit produced was actually less than the forecasted, yet the actual revenue gain from were greater than the forecasted one. This situation...
-
Dan has invested $12,000 in bonds paying 6%. How much additional money should he invest in a certificate of deposit paying 3% simple interest so that the total return on the two investments will be...
-
For the following arrangements, discuss whether they are 'in substance' lease transactions, and thus fall under the ambit of IAS 17.
-
Air is a mixture with mass percentage composition 75.5 (N 2 ), 23.2 (O 2 ), 1.3 (Ar). Calculate the entropy of mixing when it is prepared from the pure (and perfect) gases.
-
Explain the molecular origin of Raoults law and Henrys law.
-
Suppose it is found that for a hypothetical regular solution that =1.40, p A * =15.0 kPa and p B * =11.6 kPa. Draw the vapour-pressure diagram.
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


