4.0 g of oxygen gas, starting at 820°C, follow the process 1 2 shown in FIGURE P18.63....
Question:
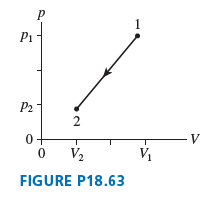
Transcribed Image Text:
P1 P2 2 0+ V2 V1 FIGURE P18.63
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (10 reviews)
Model We assume the oxygen gas is ideal Visualize From the figure w...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
A 16.0-g sample of methane (CH 4 ) reacts with 64.0 g of oxygen gas in a container fitted with a piston (at 1.00 atm and 425 K). Methane can react with oxygen to form carbon dioxide and water vapor...
-
A 1.604-g sample of methane (CH4) gas and 6.400 g of oxygen gas are sealed in a 2.50- L vessel at 411oC and are allowed to reach equilibrium. Methane can react with oxygen to form gaseous carbon...
-
Exactly 4.0 g of hydrogen gas combines with 32 g of oxygen gas according to the following reaction. 2H2 + O2 2H2O a. How many hydrogen molecules are required to completely react with 48 oxygen...
-
Discuss the different sampling procedures and techniques that you will apply to the qualitative and quantitative phases of the study.
-
On May 31, 2011, Andro Corporation sold 500 shares of its stock to Nombeko, an employee, for $100 per share. No special election is made. At the time of the sale, the fair market value of the stock...
-
Wilmington Company has two manufacturing departments-Assembly and Fabrication. It considers all of its manufacturing overhead costs to be fixed costs. The first set of data that is shown below is...
-
Jennifer Capriati, Inc. issued a $100,000, 4-year, 11% note at face value to Forest Hills Bank on January 1, 2008, and received $100,000 cash. The note requires annual interest payments each December...
-
The data identified below was listed in a projects latest status report: BCWS = $36,000 BCWP = $30,000 ACWP = $33,000 BAC = $120,000 Original length of the project 10 months Using these data,...
-
The Stockholders' Equity section of the FT Company balance sheet at the close of the current year follows: $ 6,000,000 210,000 Stockholders' Equity Preferred stock (6.3%, $100 par value, 100,000...
-
For the circuit of Fig.4.141 (a) Does VC increase or decrease if RB is increased? (b) Does IC increase or decrease if β is reduced? (c) What happens to the saturation current if...
-
Hasbro is a leading firm in the toy, game, and amusement industry. Its promoted brands group includes products from Playskool, Tonka, Milton Bradley, Parker Brothers, Tiger, and Wizards of the Coast....
-
10 g of dry ice (solid CO 2 ) is placed in a 10,000 cm 3 container, then all the air is quickly pumped out and the container sealed. The container is warmed to 0C, a temperature at which CO 2 is a...
-
Starting with Eq. (3.32), prove that the energy densities of the electric and magnet fields are equal (u E = u B ) for an electromagnetic wave. (3.32) UB 2o
-
Indicate whether each of the following types of transactions will either (a) increase stockholders' equity or (b) decrease stockholders' equity: 1. expenses 2. revenues 3. stockholders' investments...
-
The following selected transactions were completed by Lindbergh Delivery Service during October: 1. Received cash from issuing capital stock, \($75,000\). 2. Paid rent for October, \($4,200\). 3....
-
Murray Kiser operates his own catering service. Summary financial data for February are presented in equation form as follows. Each line designated by a number indicates the effect of a transaction...
-
A. Given that y = e 2x + 1 complete the table of values of y corresponding to x = 0.5, 1 and 1.5. B. Use the trapezium rule, with all the values of y in the completed table, to obtain an estimate for...
-
Draw a schematic using NFETs and PFETs for a restoring logic gate that implements the function = 0 if zero or two of inputs cba are true. Assume that all inputs and their complements are available.
-
The angle of elevation is the angle above horizontal that an observer must look to see a higher object. The angle of depression is the angle below horizontal that an observer must look to see a lower...
-
Doorharmony Company makes doorbells. It has a weighted- average cost of capital of 5% and total assets of $ 5,900,000. Doorharmony has current liabilities of $ 750,000. Its operating income for the...
-
Geiger counters are not very accurate when the count rates are very high; they indicate a count rate lower than the actual value. Explain why this is so.
-
As a general rule, the radioactivity from a particular radioisotope is considered to be reduced to a safe level after 10 half-lives have elapsed. (Obviously, the initial quantity of the isotope is...
-
The naturally occurring radioisotopes uranium-238 and uranium-235 have decay chains that end with the stable isotopes lead-206 and lead-207, respectively. Natural minerals such as zircons contain...
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


