Question: FIGURE EX38.24 is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atom??s (a) Emission spectrum (b) Absorption spectrum? n=
FIGURE EX38.24 is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atom??s
(a) Emission spectrum
(b) Absorption spectrum?
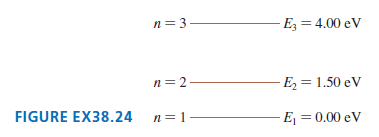
n= 3 Ez = 4.00 eV n=2- E = 1.50 eV FIGURE EX38.24 n= 1 E = 0.00 eV
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Model To conserve energy the emission and the absorption photons ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778bee6b8_700738.pdf
180 KBs PDF File
1442_6054778bee6b8_700738.docx
120 KBs Word File


