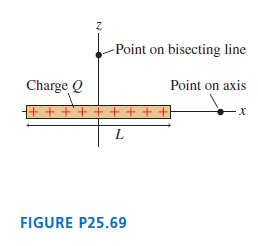
FIGURE P25.69 shows a thin rod of length L and charge Q. Find an expression for the
Question:
FIGURE P25.69 shows a thin rod of length L and charge Q. Find an expression for the electric potential a distance z away from the center of rod on the line that bisects the rod.
Transcribed Image Text:
-Point on bisecting line Charge Q Point on axis ++ + + + + L FIGURE P25.69
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Model Assume the thin rod is a line of charge with uniform linear charge density V...View the full answer

Answered By

HIMANSHU SINGH
1. Scored 86.8 percent marks in High School
2. Scored 91.2 percent marks in intermediate
3. B Tech in Chemical Engineering from IET Lucknow
4. Working experience as an Assistant Manager with TATA CHEMICALS LIMITED
5. Currently pursuing M Tech from INDIAN INSTITUTE OF TECHNOLOGY , GUWAHATI in Chemical Engineering (specialization in Petroleum Science & Technology)
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
FIGURE P23.43 shows a thin rod of length L with total charge Q. a. Find an expression for the electric field strength at point P on the axis of the rod at distance r from the center.b. Verify that...
-
FIGURE P23.44 shows a thin rod of length L with total charge Q. Find an expression for the electric field E at point P. Give your answer in component form. P FIGURE P23.44 + + + + + + + + +
-
A thin rod of length L and mass M is supported in a horizontal position by two strings, one attached to each end as shown in Figure. If one string is cut, the rod begins to rotate about the point...
-
A. What is the expected dividend in two years? Suppose NI = $85,000 B. What is Samsung's WACC? Samsung's capital structure is 65% ordinary equity and 35% debt. He has operating assets equal to...
-
What social responsibility obligations should too-level management of major retailers have for the severe injuries that often take place in their stores on Black Friday? (This is the highly-promoted...
-
For the last five years, a firm has measured (in euros) the following quality costs by categories: a. Given these costs, is it likely that the companys defective rate has gone up or down? Explain. b....
-
Use data tables to conduct simple Monte Carlo simulations.
-
Western, Corp., produces two products, cigars and chewing tobacco, from a joint process involving the processing of tobacco leaves. Joint costs are $60,000 for this process, and yield 2,000 pounds of...
-
Dode Industries has hired the investment banking firm of Eric, Schwartz, & Mann (ESM) to help it go public. Dode and ESM agree that Dode's current value of equity is $55 million. Dode currently has 4...
-
Draw the graph of the NRZ-L scheme using each of the following data streams, assuming that the last signal level has been positive. From the graphs, guess the bandwidth for this scheme using the...
-
The concentration of carbon monoxide (CO) in a gas sample is measured by a spectrophotometer and found to be 85 ppm. Through long experience with this instrument, it is believed that its measurements...
-
FIGURE P25.71 shows a thin rod with charge Q that has been bent into a semicircle of radius R. Find an expression for the electric potential at the center. Center Charge Q
-
a. What is the primary goal of investor-owned corporations? b. What is the primary goal of most not-for-profit healthcare corporations? c. Are there substantial differences between the finance goals...
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
Perform the indicated operations. 4ax 3 - 8ax 4 /-2ax
-
Is that Yelp review real or fake? The article A Framework for Fake Review Detection in Online Consumer Electronics Retailers (Information Processing and Management 2019: 12341244) tested five...
-
If you wanted to slow down the chain reaction in a nuclear reactor, would you remove or insert the control rods? Explain.
-
Will a reactor that uses ordinary water as the moderator be able to operate using unenriched uranium as a fuel? Explain.
-
If a reactor goes subcritical, will the chain reaction speed up? Explain.
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


