Based on Table 1, what is the relationship between decay energy and the type of particle emitted?
Question:
Based on Table 1, what is the relationship between decay energy and the type of particle emitted?
F. Beta particles tend to have higher decay energies.
G. Alpha particles tend to have lower decay energies.
H. Alpha particles tend to have higher decay energies.
J. There is no apparent relationship between type of particle and decay energy.
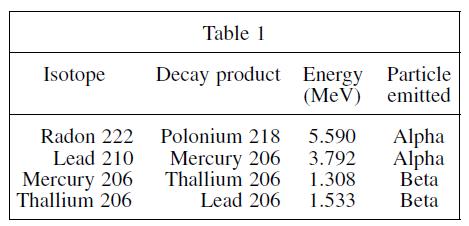
Transcribed Image Text:
Isotope Radon 222 Lead 210 Mercury 206 Thallium 206 Table 1 Decay product Energy Particle (MeV) emitted Polonium 218 Mercury 206 Thallium 206 Lead 206 5.590 3.792 1.308 1.533 Alpha Alpha Beta Beta
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The best answer is H According to Table 1 the energies associated with alpha particle e...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Business questions
-
1. The famous capital asset pricing model or CAPM is a single factor model that characterizes the portion of an assets total return that results from systematic movements in the broader market. It is...
-
What is the relationship between the price of crude oil and the price you pay at the pump for gasoline? The file Oil & gas contains the price ($) for a barrel of crude oil (Cushing, Oklahoma spot...
-
What is the relationship between the price of crude oil and the price you pay at the pump for gasoline? The file Oil & Gasoline contains the price (S) for a barrel of crude oil (Cushing, Oklahoma,...
-
Determine whether each of these statements is true or false. a) x {x} b) {x} {x} c) {x} {x} d) {x} {{x}} e) {x} f) {x}
-
At the end of October, Santa Fe Companys management estimates the uncollectible accounts expense to be 1 percent of net sales of $1,385,000. Prepare the journal entry to record the uncollectible...
-
Simplify. Assume that all variables represent positive real numbers. V6. VII 11
-
How are distributions from S corporations compared to dividends from C corporations?
-
A fuel gas containing 85.0 mole% methane and the balance ethane is burned completely with pure oxygen at 25C and the products are brought back down to 25C. (a) Suppose the reactor is continuous. Take...
-
18. During 2021, Cinnamon Buns Co. (CBC) recorded the following transactions relating to its inventories (listed in chronological order) January 1 Beginning inventory 70 units @ $55 May 10 Purchase...
-
According to the passage, which of the following was the independent variable in each of the studies? F. The surface upon which the golf cart was driven. G. The amount of fuel in the tank of the golf...
-
A criticism of this study is that the order that the grackles were placed in the cage may have affected the aggressiveness of each bird. The best way to refute this criticism would be to: A....
-
All measures of variation are nonnegative in value for all sets of data. a. What does it mean for a value to be nonnegative? b. Describe the conditions necessary for a measure of variation to have...
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
M. Munir Company would like to design, produce, and sell versatile toasters for the home kitchen market. The toaster will have four slots that adjust in thickness to accommodate both slim slices of...
-
Solve each equation or inequality. |6x8-4 = 0
-
Acorn Corporation is publicly traded on the American Stock Exchange. Its chief executive officer, Carl, currently receives an annual salary of $1 million. The board of directors is considering...
-
Current tax law imposes a $1 million limitation on the deductibility of executive compensation. Should such limitation be retained or repealed? Give reasons for your opinion.
-
The current corporate tax structure contains phase-outs (via surtaxes) at various levels of taxable income that eliminate the benefits of lower tax brackets. Identify the two phase-outs in the...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


