Based on the results of Experiment 1, if a solution with a concentration of 1.5 ppm sulfite
Question:
Based on the results of Experiment 1, if a solution with a concentration of 1.5 ppm sulfite had been tested, the corrected absorbance would have been closest to which of the following values?
A. 0.160
B. 0.240
C. 0.300
D. 0.360
Experiment 1
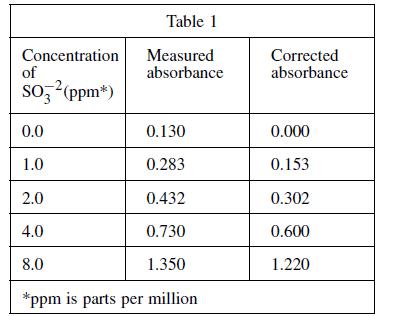
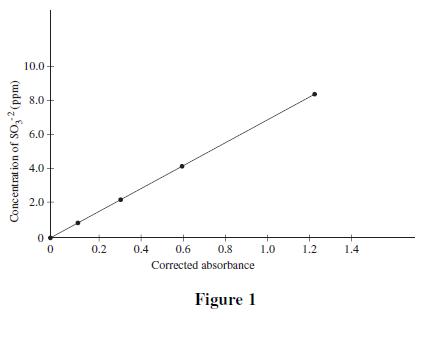
Four solutions, each containing a different amount of sulfite dissolved in H2O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).
Step by Step Answer:






