Answered step by step
Verified Expert Solution
Question
1 Approved Answer
So far, we've only looked at some simple elimination reactions where only one product is possible. In this post we'll look at some examples
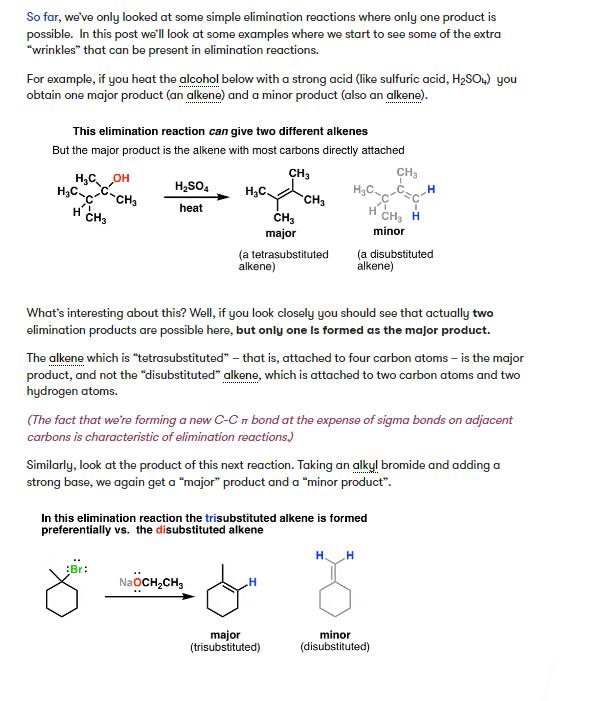

So far, we've only looked at some simple elimination reactions where only one product is possible. In this post we'll look at some examples where we start to see some of the extra "wrinkles" that can be present in elimination reactions. For example, if you heat the alcohol below with a strong acid (like sulfuric acid, HSO4) you obtain one major product (an alkene) and a minor product (also an alkene). This elimination reaction can give two different alkenes But the major product is the alkene with most carbons directly attached CH3 CH3 HCOH HCC-CH3 H2SO4 heat HC HC H CH3 H CH3 CH3 CH H minor major (a tetrasubstituted alkene) (a disubstituted alkene) What's interesting about this? Well, if you look closely you should see that actually two elimination products are possible here, but only one is formed as the major product. The alkene which is "tetrasubstituted" - that is, attached to four carbon atoms - is the major product, and not the "disubstituted" alkene, which is attached to two carbon atoms and two hydrogen atoms. (The fact that we're forming a new C-C bond at the expense of sigma bonds on adjacent carbons is characteristic of elimination reactions.) Similarly, look at the product of this next reaction. Taking an alkyl bromide and adding a strong base, we again get a "major" product and a "minor product". In this elimination reaction the trisubstituted alkene is formed preferentially vs. the disubstituted alkene :Br: H. H " NaOCH2CH3 H major (trisubstituted) minor (disubstituted) Again, the major product is "more substituted" than the minor product. Of the 4 atoms directly attached to the alkene in the major product, 3 of them are carbon and 1 is hydrogen. In the minor product, 2 carbon atoms and 2 hydrogen atoms are directly attached to the alkene. ......... So what's responsible for this preference for the "more substituted" alkene in elimination reactions?
Step by Step Solution
★★★★★
3.23 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
In both examples the major product of the elimination reaction is the alkene with the most substituted double bond This means that the major product is the one where the double bond is attached to mor...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


