(a) Explain why the diffusion coefficient of CH 3 OH is greater than that of sucrose in...
Question:
(a) Explain why the diffusion coefficient of CH3OH is greater than that of sucrose in Table 23-1.
(b) Make an order-of-magnitude estimate of the diffusion coefficient of water vapor in air at 298 K.
Table 23-1
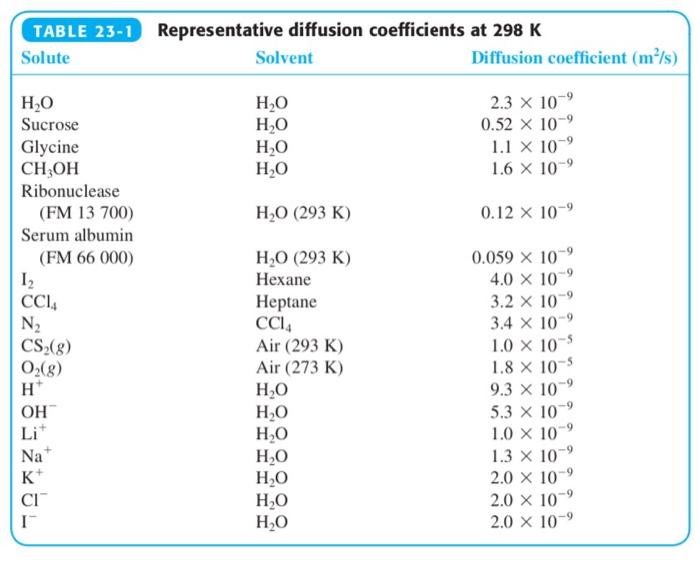
Transcribed Image Text:
TABLE 23-1 Representative diffusion coefficients at 298 K Solute Solvent Diffusion coefficient (m/s) 2.3 x 10 0.52 x 10 H,O H,O H,0 H,O H,O Sucrose Glycine CH,OH 1.1 X 10 1.6 X 10-9 Ribonuclease (FM 13 700) H,O (293 K) 0.12 x 10-9 Serum albumin (FM 66 000) Н.О (293 К) 0.059 X 10 4.0 x 10 3.2 x 10-9 Hexane CCI, N2 CS-(g) O(g) H* Неptane CCI, Air (293 K) Air (273 K) 3.4 x 10 1.0 x 10 1.8 x 10-5 9.3 x 10 5.3 x 10 H,0 H,0 H,0 H,O H,0 H,O H,O OH Li* 1.0 X 10 1.3 x 10 2.0 x 10 2.0 x 10-9 2.0 x 10-9 Na+ K+ CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a The diffusion coefficient of CH3OH is greater than that of sucrose in Table 231 because CH3OH has ...View the full answer

Answered By

Upasana Nasa
I am a dedicated and hardworking tutor committed to ensuring the success of my students. I am an experienced tutor who has tutored for the last 3 years, which gives me broad experience as a tutor and enable's me to tailor my style to the specific needs of a student. I provide step by step explanation of any problem to enable a student to follow, learn, and understand how to work on the problem on their own.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. The diffusion coefficient of sucrose in water at 298 K is 0.522 10 9 m 2 s 1 . Determine the time it will take a sucrose molecule on average to diffuse an rms distance of 1 mm. b. If the molecular...
-
A laboratory apparatus to measure the diffusion coefficient of vapor-gas mixtures consists of a vertical, small-diameter column containing the liquid phase that evaporates into the gas flowing over...
-
The diffusion coefficient of I in hexane at 25C is 4.05 x 10-9 m2 S-1. Estimate the time required for an iodine molecule to have a root mean square displacement of 1.0 cm.
-
The 32-kg spool of outer radius r, = 420 mm has a centroidal radius of gyration k = 265 mm and a central shaft of radius r; = 155 mm. The spool is at rest on the incline when a tension T= 243 N is...
-
How is workforce plans related to business and HR strategies?
-
A pitched flat roof truss is loaded as shown. Determine the force in members EG, GH, and HJ. 2 kips 4 kips 4 kips 4 kips 2 kips 1.5 ft 7.5 ft EJ 7 ft 7ft 7 ft
-
A random sample of five pairs of observations were selected, one of each pair from a population with mean p,, the other from a population with mean pp The data are shown in the accompanying table....
-
In the early 1970s a widely publicized list of the "Nifty Fifty" stocks was drawn up. This list, which included Avon Products, Polaroid, Coca-Cola, McDonald's, Walt Disney, American Express, and...
-
QUESTION 1 1. The bonds issued by Jensen & Son bear a 6 percent coupon, payable semiannually . The bond matures in 8 years and has a $1,000 face value. Currently, the bond sells at par. What is the...
-
An LP model is expressed in terms of its technology matrix (A), RHS vector(b), and cost vector (c) in an Excel file named 'Params.xlsx' with 3 sheets (attached). We would like to minimize the...
-
Consider the titration of 100.0 mL of 0.010 0 M Ce 4+ in 1 M HClO 4 by 0.040 0 M Cu + to give Ce 3+ and Cu 2+ , using Pt and saturated Ag | AgCl electrodes to find the end point. (a) Write a balanced...
-
A 50.00-mL sample containing La 3+ was treated with sodium oxalate to precipitate La2(C 2 O 4 ) 3 , which was washed, dissolved in acid, and titrated with 18.04 mL of 0.006 363 M KMnO 4 . Write the...
-
Due to the significance of the information theyre trusted with, management accountants are expected to observe well-defined professional ethical standards. Professional management accountant...
-
Presented below is information related to Rembrandt Inc's inventory, assuming Rembrandt uses lower-of-LIFO cost- or-market. (per unit) Skis Boots Parkas Historical cost $190.00 $106.00 $53.00 Selling...
-
7. Below is a UML model describing a typical organization of class modules taken by students: A student can take several modules. (Note that a module is offered even if it is not taken by any...
-
Beswick Limited manufactures mountain and road bikes. The trial balance at 3 1 December 2 0 2 0 was as follows: Dr Cr Revenue 3 , 5 6 4 , 3 0 0 Purchases 1 , 5 7 8 , 2 5 0 Inventory on 3 1 / 1 2 / 1...
-
Kubin Company's relevant range of production is 10,000 to 12,000 units. When it produces and sells 11,000 units, its average costs per unit are as follows: Average Cost per Unit $ 7.10 Direct...
-
Smithen Company, a wholesale distributor, has been operating for only a few months. The company sells three products-sinks, mirrors, and vanities. Budgeted sales by product and in total for the...
-
Janet purchased her personal residence in 2009 for $250,000. In January 2019 she converted it to rental property. The fair market value at the time of conversion was $210,000. a. Determine the amount...
-
Given that all the choices are true, which one concludes the paragraph with a precise and detailed description that relates to the main topic of the essay? A. NO CHANGE B. Decades, X-ray C. Decades...
-
Find the pH of 0.050 M NaCN.
-
Calculate the fraction of association () for 1.00 10-1, 1.00 10-2, and 1.00 1012 M sodium acetate. Does increase or decrease with dilution?
-
A 0.10 M solution of a base has pH = 9.28. Find Kb.
-
Need help filling out these tax forms. Not sure how to do 1040 page 2 or schedule 3. I think I have schedule 1 right but need help with the itemized deductions for 1040 page 1 Required information...
-
Question:What should Airbus and Boing have learned from IBERIA case? What changed in the industry when Boing decided to develop Dreamliner in 2003?( Read the following case and ppt) Airline Route...
-
Which of the following needs to be always assessed when you are evaluating the literature you have obtained for your research? O All of the above O Sufficiency Value O Relevance Several approaches...

Study smarter with the SolutionInn App


