A mixture containing only aluminum tetrafl uoroborate, Al(BF 4 ) 3 (FM 287.39), and magnesium nitrate, Mg(NO
Question:
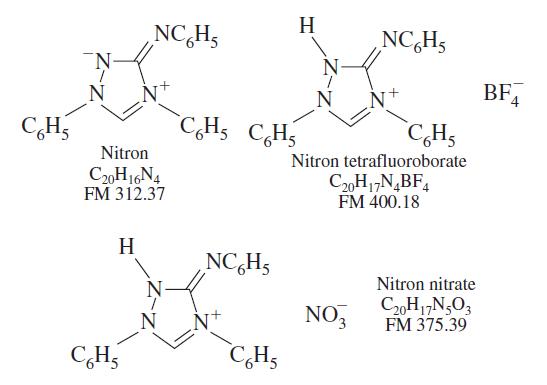
A mixture containing only aluminum tetrafl uoroborate, Al(BF4)3 (FM 287.39), and magnesium nitrate, Mg(NO3)2 (FM 148.31), weighed 0.282 8 g. It was dissolved in 1 wt% HF(aq) and treated with nitron solution to precipitate a mixture of nitron tetrafluoroborate and nitron nitrate weighing 1.322 g. Find the wt% Mg in the original solid mixture.
Transcribed Image Text:
H NC,Hs NC,H5 BF, CH5 C,Hs CH5 CH; Nitron Nitron tetrafluoroborate C20H16N4 FM 312.37 C2,H1,N,BF, FM 400.18 H NC,H5 Nitron nitrate NO, C20H17N,O3 FM 375.39 N CH, C,H,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Nitronis C 20 H 16 N 4 Nitron tetrafluoroborate is C 20 H 16 N 4 BF 4 FM 39918 Nitro...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A mixture containing only Al2O3 (FM 101.96) and Fe2O3 (FM 159.69) weighs 2.019 g. When heated under a stream of H2, Al2O3 is unchanged, but Fe2O3 is converted into metallic Fe plus H2O(g). If the...
-
A solid mixture weighing 0.5485 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1M H2SO4, oxidized to Fe3+ with H2O2, and...
-
A mixture weighing 7.290 mg contained only cyclohexane, C6H12(FM 84.159), and oxirane, C2H4O (FM 44.053). When the mixture was analyzed by combustion analysis, 21.999 mg of CO2 (FM 44.010) were...
-
A force F = (3.00 N)i + (7.00 N)j + (7.00 N)k acts on a 2.00 kg mobile object that moves from an initial position of di = (3.00 m)i (2.00 m)i + (5.00 m)k to a final position of df = (5.00 m)i +...
-
Should discussions of employee job performance be separated from salary considerations?
-
The 200 200-mmsquare plate shown has a mass of 25 kg and is supported by three vertical wires. Determine the mass and location of the lightest block which should be placed on the plate if the...
-
In education, the term instructional technology refers to products such as computers, spreadsheets, CD-ROMs, vidcos, and presentation software. How frequently do professors use instructional...
-
According to the case above, advise Mr. Doolittle as to whether the expenses incurred in the United Kingdom would be deductible for income tax purposes? You are a chartered tax accountant working in...
-
Calculating and interpreting current yield and yield to maturity: Describe and differentiate between a bonds (a) current yield and (b) yield to maturity. Why are these yield measures important to the...
-
ws EX 16-17 Statement of cash flows OBJ. 5 The comparative balance sheet of Hirayama Industries Inc. for December 31, 2012 and 20Y1, is as follows: Cash.... Accounts receivable (net).. Inventories.....
-
Why is high relative supersaturation undesirable in a gravimetric precipitation?
-
Explain what is done in thermogravimetric analysis.
-
Johnson Corporation's bank statement for October reports an ending balance of $6,248, whereas Johnson's cash account shows a balance of $5,680 on October 31. The following additional information is...
-
Question 7 Two objects, of masses 3 and 4 kg, are hung from the ends of a stick that is 70 cm long and has marks every 10 cm, as shown above. If the mass of the stick is negligible, at which of the...
-
Since they do not have enough saved, Rachel and John would like to consider retiring later. Create a new timeline and recalculate all of the relevant values to determine at what age Rachel and John...
-
Problem 6 Find the partial derivative with respect to x for the following functions: (a) p = 56 (b) y(x)=56-4x (c) m = r (d) q= x (e) f(x) =x3 (f) g(x,y) = xy 2 (g) h(x,y) = Ax1/2y1/2, where A is a...
-
Consider the information in the file named Cost Functions of the Firm (also presented above). Please read that file carefully before answering this and the following questions. The fixed cost of...
-
On January 1, 2022, Monica Company acquired 80 percent of Young Company's outstanding common stock for $872,000. The fair value of the noncontrolling interest at the acquisition date was $218,000....
-
Jennifer and Paul, who file a joint return, have taxable income of $82,825 and the following tax liability: $19,400 10% = $ 1,940.00 ($78,950 $19,400) 12% = 7,146.00 ($82,825 $78,950) 22% =...
-
The following processes constitute the air-standard Diesel cycle: 12: isentropic compression,23: constant-volume energy addition (T and P increase),34: constant-pressure energy addition (v...
-
State whether the errors in (a) - (d) are random or systematic: (a) A 25-mL transfer pipet consistently delivers 25.031 0.009 mL. (b) A 10 - mL buret consistently delivers 1.98 0.01 mL when drained...
-
Cheryl, Cynthia, Carmen, and Chastity shot the targets below at Girl Scout camp. Match each target with the proper description. (a) Accurate and precise (b) Accurate but not precise (c) Precise but...
-
Rewrite the number 3.123 56 ( 0.167 89%) in the forms (a) number ( absolute uncertainty) and (b) number ( percent relative uncertainty) with an appropriate number of digits.
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...
-
Built-Tight is preparing its master budget for the quarter ended September 30, 2015. Budgeted sales and cash payments for product costs for the quarter follow: July August September Budgeted sales $...
-
inepired. 2. Suppliei on hard at the eind of the month tedaled $16800. 2 The balance in Prepaid Rent represucks 4 months of rent coves. 5. Desreciationet bullines is $5060 per vear

Study smarter with the SolutionInn App


