Cathodic stripping of ClO -4 in Figure 17-26 does not involve oxidation or reduction of ClO -4
Question:
Cathodic stripping of ClO-4 in Figure 17-26 does not involve oxidation or reduction of ClO-4. Explain how this measurement works.
In Figure 17-26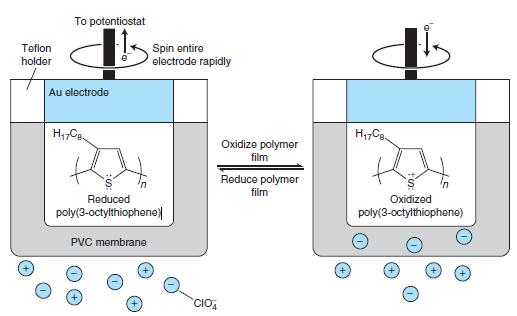
Transcribed Image Text:
To potentiostat Teflon Spin entire electrodo rapidly holder Au electrode Oxidize polymor film Reduce polymer film Reduced Oxidized poly(3-octylthiophene) poly(3-octylthiophene) PVC membrane CIO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
An electrode is dipped into the solution The electrode is connected to a power supply and a current ...View the full answer

Answered By

Jemima Wangu
I am a proficient tutor with the following skills;
Effective time management
Microsoft Office
Computer skills
Multitasking skills
Strong Mathematical skills
Honesty and integrity
Advanced technology skills
Motivated attitude
Content writing
Content research and development
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why a census does not necessarily have to involve a population of people. Use an example to illustrate.
-
Explain how calorimetry works to calculate H or E for a reaction. Does the temperature of the calorimeter increase or Decrease for an endothermic reaction? How about for an exothermic reaction?...
-
Does this reduction appear to be constant, accelerating, or decelerating? Explain.
-
Divalent carbon species called carbenes care capable of fleeting existence. For example, methylene: CH2, is the simplest carbene. The two unshared electrons in methylene can be either spin-paired in...
-
Suppose you were asked to enter a debate in which your task was to argue against any special effort to manage workforce diversity. What would you say?
-
The 192-N load can be moved along the line of action shown and applied at A, D, or E. Determine the components of the reactions at B and F when the 192-N load is applied (a) At A, (b) At D, (c) At E....
-
In forming real estate portfolios, investors typically diversify the geographic locations of their holdings. While some choose to own properties in a variety of different metropolitan areas, others...
-
Jonsub Ltd. is a wholly owned subsidiary of Normpar Ltd. Normpar Ltd. plans to merge the two companies, either by amalgamating with the subsidiary [sec. 87] or by winding up the subsidiary [ssec....
-
Jarvene Corporation uses the FIFO method in its process costing system. The following data are for the most recent month of operations in one of the company's processing departments: Units in...
-
The following information is available for Park Valley Spa for July Year 1: The following is a list of checks and deposits recorded on the books of the Park Valley Spa for July Year 1: Other...
-
In 1 M NH 3/1 M NH 4 Cl solution, Cu 2+ is reduced to Cu+ near 20.3 V (versus S.C.E.), and Cu + is reduced to Cu(in Hg) near 20.6 V. (a) Sketch a qualitative sampled current polarogram for a solution...
-
Vapor at a pressure of 30.3 mbar from the solid compound pyrazine had a transmittance of 24.4% at a wavelength of 266 nm in a 3.00-cm cell at 298 K. (a) Convert transmittance to absorbance. (b)...
-
Draw the EITC diagram, and put in round numbers for a family of four. Explain your diagram.
-
Which one of the following therapists' approaches has been integrated into several other therapies in the West? 1. Naikan therapy 2. Morita therapy 3. mindfulness meditation
-
What does this scatter plot tell us? Check ALL below that are true from the Scatter Plot. Y is cumulative total barrels and x is number of active wells. 1. If there were 550 active wells, we would...
-
Millie runs a small company that makes customised notebooks with personalised details on the cover and inserts. You promote your product as a great gift idea, and your holiday orders break your...
-
Excel ACC 311 Project Two Workbook Template - View-only Search (Alt + Q) File Home Insert Draw Page Layout Formulas Data Review View Help 12 B A ... ab Ev F10 1 2 3 4 5 6 fx A B Posey's Pet Emporium...
-
Imagine that you are the change manager for acompany that does business entirely via the Internet. The head development engineercalls to indicate he wants to make a small change to one of the...
-
On April 15, 2017, Andy purchased some furniture and fixtures (7-year property) for $10,000 to be used in his business. He did not elect to expense the equipment under 179 or bonus depreciation. On...
-
Given find the value of k. es 1 e kx dx = 1 4'
-
(a) Calculate the pH of a solution prepared by mixing 0.0100 mol of the base B (Kb = 10 = - 2.00) with 0.020 0 mol of BH+Br- and diluting to 1.00 L. First calculate the pH by assuming [B] = 0.0100...
-
Effect of ionic strength on pKa. Ka for the H2PO4- /HPO24- buffer is If you mix a 1:1 mole ratio of H2PO-4 and HPO42- at 0 ionic strength, the pH is 7.20. Using activity coefficients from Table 7-1,...
-
Systematic treatment of equilibrium. The acidity of Al3+ is determined by the following reactions. Write the equations needed to find the pH of Al(ClO4)3 at a formal concentration F. 2 + 2H20-Al(...
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


