Question: Explain why each voltammogram from the rotating disk electrode in Figure 17-16 reaches a plateau at low potential and at high potential. What chemistry occurs
Explain why each voltammogram from the rotating disk electrode in Figure 17-16 reaches a plateau at low potential and at high potential. What chemistry occurs in each plateau? Why do all the curves overlap at low potential? How might the current density in each plateau change if the rotation speed were decreased?
In Figure 17-16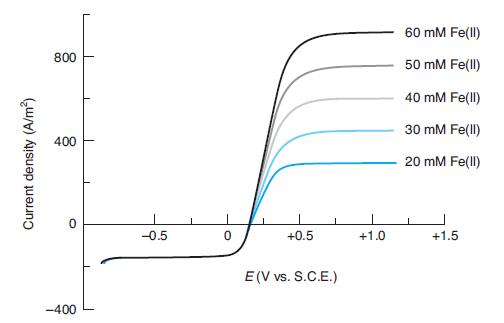
60 mM Fe(ll) 800 50 mM Fe(ll) 40 mM Fe(ll) 30 mM Fe(ll) 400 20 mM Fe(ll) -0.5 +0.5 +1.0 +1.5 E(V vs. S.C.E.) -400 Current density (A/m)
Step by Step Solution
3.37 Rating (169 Votes )
There are 3 Steps involved in it
Each volt amm ogram reaches a plateau at low potential beca... View full answer

Get step-by-step solutions from verified subject matter experts


