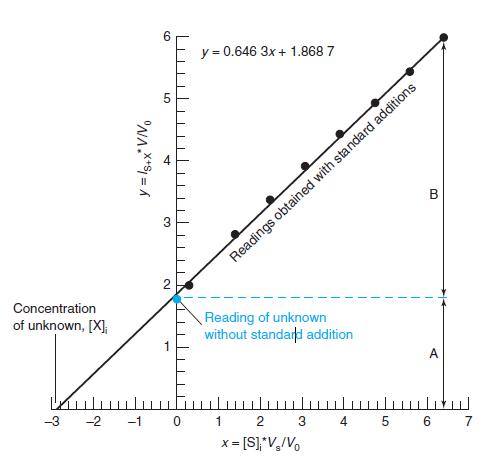
In Figure 5-6, the x-intercept is 22.89 mM and its standard uncertainty is 0.098 mM. Find the
Question:
In Figure 5-6, the x-intercept is 22.89 mM and its standard uncertainty is 0.098 mM. Find the 90% and 99% confi dence intervals for the intercept.
Transcribed Image Text:
y = 0.646 3x + 1.868 7 В Readings obtained with standard additions 2 Concentration of unknown, [X], Reading of unknown without standard addition A -3 -2 -1 0 1 2 3 4. 5 6 7 x = [S]*V,/Vo LO 4.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Given xintercept 2289 mM standard uncertainty 0098 mM We can use the tdistribution to find the ...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
Find the uncertainty in Y, given that X = 4.0 0.4 and a. Y = X2 b. Y =X c. Y = 1/X d. Y = ln X e. Y = eX f. Y = sin X (X is in units of radians)
-
Find intervals containing solutions to the following equations. a. x 3x = 0 b. 4x2 ex = 0 c. x3 2x2 4x + 2 = 0 d. x3 + 4.001x2 + 4.002x + 1.101 = 0
-
In 1987, British skier Graham Wilkie achieved a speed of v = 211 km/h going downhill. Assuming that he reached the maximum speed at the end of the hill and then continued on the horizontal surface,...
-
How might the Internet change the ways that employees and managers interact?
-
When the sun is either rising or setting and appears to be just on the horizon, it is in fact below the horizon. The explanation for this seeming paradox is that light from the sun bends slightly...
-
A study reported in the Journal o,f Psychology (Mar. 1991) measures the change in female students' selfconcepts as they move from high school to co1lege.A sample of 133 Boston College first-year...
-
National Bank operates a network of automated teller machines (ATMs). Cash withdrawals at an ATM aver-age about $ 80. The bank estimates that the fixed cost of filling an ATM with cash is about $ 100...
-
Market value of the shares are decided by__.choose correct option and explain and also explain incorrect options and also explain summary Athe respective companies Bthe investment market Cthe...
-
Whitegloves Janitorial Service was started 2 years ago by Lynn Sanders. Because business has been exceptionally good, Lynn decided on July 1, 2019, to expand operations by acquiring an additional...
-
Explain the meaning of the quotation at the beginning of this chapter: Get the right data. Get the data right. Keep the data right.
-
Internal standard graph. Data are shown below for chromatographic analysis of naphthalene (C 10 H 8 ), using deuterated naphthalene (C 10 D 8 , in which D is the isotope 2H) as an internal standard....
-
In Prob. 3.60 find the torque caused around flange 1 if the center point of exit 2 is 1.2 m directly below the flange center. CV 40
-
what extent do you perceive that your personal values align with the core ethos and culture of the organization?
-
Safe, avoidant, indecisive, and disorganized. What attachment style do you believe you grew up with, and how did it affect your cognitive and personality development as a child? Think about the types...
-
What do you think about an 'employee-centric' rather than an 'employer-centric' PMS. Which would work better in your current (or prior) organization? Make sure to provide specific examples to justify...
-
How do I relate the below case study to RLR - Responsible Leadership for Relations? Relate and analyses in detail....
-
How do advanced integrative approaches, combining elements of cognitive-behavioral therapy, mindfulness, and somatic experiencing, offer comprehensive solutions for addressing the multifaceted nature...
-
In 2015, Chara incurred a loan to pay for qualified higher education expenses for her 20-year-old daughter who was a dependent. In 2019, her granddaughter graduated from college, moved away to start...
-
Write a paper about the Working relationship in the organization- collaboration within and outside the organization
-
How many milliliters of 0.0500 M EDTA are required to react with 50.0 mL of 0.0100 M Ca2+? With 50.0 mL of 0.0100 M Al3+?
-
A 50.0-mL sample containing Ni2+ was treated with 25.0 mL of 0.050 0 M EDTA to complex all the Ni2+ and leave excess EDTA in solution. The excess EDTA was then back titrated, requiring 5.00 mL of...
-
A 50.0-mL aliquot of solution containing 0.450 g of MgSO4 (FM 120.37) in 0.500 L required 37.6 mL of EDTA solution for titration. How many milligrams of CaCO3 (FM 100.09) will react with 1.00 mL of...
-
TB SA Qu. 13-74 (Static) What must Abdu invest today to... What must Abdu invest today to receive an annuity of $9,000 for four years semiannually at an 8% annual rate? All withdrawals will be made...
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...

Study smarter with the SolutionInn App


