Write a balanced chemical equation (in acidic solution) for the reaction represented by the question mark.22 As
Question:
Write a balanced chemical equation (in acidic solution) for the reaction represented by the question mark.22 As in Box 14-5, calculate Eo for the reaction.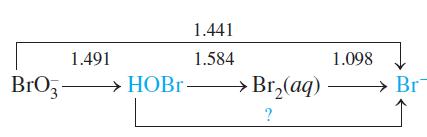
Transcribed Image Text:
1.441 1.491 1.584 1.098 BrO,- → HOB - НОВГ- Br,(aq) - » Br,(aq) Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)

Answered By

Manish kumar
Hello everyone! My name is ManishKumar, and I am thrilled to be your teacher I wanted to take a moment to introduce myself and share a little bit about my background.
I have always been passionate about education and helping students reach their full potential. I have 5 years of experience in teaching and have had the privilege of working with diverse groups of students throughout my career.
I hold a [Bachelor's/Master's/Ph.D.] degree in from Himachal PradeshUniversity, where I gained a deep understanding of the subject and developed effective teaching strategies. I believe that education is a lifelong journey, and I continue to expand my knowledge and skills through professional development programs and staying up to date with the latest educational research and practices.
In my classroom, I strive to create a positive and inclusive learning environment where every student feels valued and supported. I believe in fostering a collaborative and engaging atmosphere, encouraging active participation, critical thinking, and creativity.
Apart from teaching, I have a passion for music . I believe that these personal interests help me bring a unique perspective to the classroom and connect with students on a deeper level.
I am excited to embark on this learning journey with all of you and look forward to getting to know each and every one of you. Please feel free to reach out to me if you have any questions, concerns, or if you simply want to chat. Let's make this a memorable and successful journey together!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a balanced chemical equation for the neutralization reaction between each given acid and base. Include the proper phase labels. a. HI(aq) + KOH(aq) ( ? b. H2SO4(aq) + Ba(OH)2(aq) ( ?
-
Write a balanced chemical equation for the neutralization reaction between each given acid and base. Include the proper phase labels. a. HNO3(aq) + Fe(OH)3(s) ( ? b. H3PO4(aq) + CsOH(aq) ( ?
-
Write a balanced chemical equation for each of the following reactions: (a) Phenol + sodium hydroxide (b) Product of part (a) + ethyl bromide (c) Product of part (a) + butyl p-toluenesulfonate (d)...
-
A charged isolated metal sphere of diameter 10 cm has a potential of 8000 V relative to V = 0 at infinity. Calculate the energy density in the electric field near the surface of the sphere.
-
What is meant by the statement that training is extremely "faddish"?
-
Determine by direct integration the centroid of the area shown. Express your answer in terms of a and b. * = ky2 9.
-
Refer to the Working Women (June 1999) comparison of the percentages of adult Americans in 1975 and 1999 who would vote for a woman president. Exercise
-
The Questron Company manufactures telecommunications equipment at its plant in Scranton, Pennsylvania. The company has marketing divisions throughout the world. A Questron marketing division in...
-
Question 6 McPherson furniture Company started construction of a combination office and warehouse building for its own use at an estimated cost of 5,000,000 on January McPherson expected to complete...
-
Distinguish among the value chain, the supply chain, and the distribution chain.
-
(a) Write the Nernst equations for the half-reactions in Demonstration 14-1. In which direction do electrons move through the circuit? (b) If you use your fingers as a salt bridge in Demonstration...
-
What must be the relation between E o+ and E o- if the species X+ is to diproportionate spontaneously under standard conditions to X 3+ and X(s)? Write a balanced equation for the disproportionation.
-
How well does a students Verbal SAT score (on an 800-point scale) predict future college grade point average (on a four-point scale)? Computer output for this regression analysis is shown, using the...
-
Current Attempt in Progress The adjusted trial balance of Anthony Co. for the year ending December 31, 2025, contains the following. Anthony Co. Adjusted Trial Balance December 31, 2025 Debit Credit...
-
The coefficient of performance (COP) for a heat pump used as a heater (of a house, for example) is defined as 0=-QH/W, the ratio of the total heat flow -QH into the hot place (the house) to the work...
-
6 . A cylindrical furnace is operating at a temperature of 1 2 0 0 K and is emitting radiation uniformly in all directions. The inside diameter of the furnace is 2 m and the length of the furnace is...
-
How trade creates value ( Chapter 2 ) Max Daily Production Steaks Shrimp ( lbs . ) Fry Daddy 5 0 2 0 0 Grill Master 4 0 8 0 Refer to the above production data table for Fry Daddy and Grill Master....
-
Compounds A and B have the following vapor pressures: 150 o F: PA=600mmHg PB=500mmHg 200 o F: PA=1000mmHg PB=950mmHg Assume that these compounds form ideal solution, calculate the...
-
Michael paid $3,350 in foreign income taxes to Argentina. His total worldwide income was $75,000, which included $9,800 of income from Argentina. His U.S. tax liability is $18,750. How much can...
-
Solve each equation. x 3 - 6x 2 = -8x
-
Consider the linear calibration curve, which is derived from the 14 corrected absorbances in the shaded region at the right side of. Create a least-squares spreadsheet like Figure 4 - 15 to compute...
-
Here are mass spectrometric signals for methane in H2: a) Subtract the blank value (9.1) from all other values. Then use the method of least squares to find the slope and intercept and their...
-
Nonlinear calibration curve. Following the procedure in Box 4 - 2, find how many micrograms (g) of protein are contained in a sample with a corrected absorbance of 0.350.
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


